A healthy gut is a multi-species society: it is the cooperative product of the human body with trillions of bacterial cells from a thousand or more species.
An unhealthy gut is, more often than not, the product of a breakdown in this collaboration. Often, it is triggered by displacement of cooperative, commensal species of bacteria by pathogenic bacteria, fungi, viruses, and protozoa. This is why a long course of antibiotics, killing commensal bacteria, is often the prelude to bowel ailments.
It is difficult for the immune system to defeat gut infections without the help of commensal bacteria. Think about what the immune system has to deal with. The ulcers in ulcerative colitis are essentially the equivalent of infected skin abscesses, but in the colon. Here is a description of a bowel lesion in Crohn’s disease:
Ileal lesions in Crohn’s disease (CD) patients are colonized by pathogenic adherent-invasive Escherichia coli (AIEC) able to invade and to replicate within intestinal epithelial cells. [1]
Now imagine an infected skin abscess, but with feces spread over it three times a day, or stomach acid and digestive enzymes. How quickly would you expect it to heal?
Commensal “probiotic” bacteria are like a mercenary army fighting on behalf of the digestive tract. By occupying the interior lining of the digestive tract, they deprive pathogens of a “home base” that is sheltered from immune attack. If commensal bacteria dominate the gut, the immune system can usually quickly defeat infections.
This suggests that introduction of probiotic bacteria to the gut should be therapeutic for bowel disease.
Probiotic Supplements Are Inadequate
Most supermarket probiotics contain Lactobacillus or Bifidobacterium species. These species are specialized for digesting milk; they populate the guts of infants as they start breastfeeding, and are used by the dairy industry to ferment cheeses and yogurt.
These supplements are very effective at fighting acute diarrhea from most food-borne infections. A fistful of probiotic capsules taken every hour will usually quickly supplant the pathogens and end diarrhea.
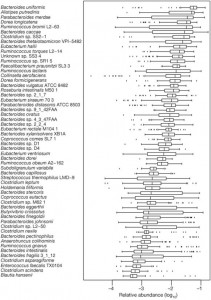
However, against more severe bowel diseases caused by chronic infections and featuring damaged intestinal mucosa, these species are usually not helpful. One issue is that they provide only a tiny part of a healthful adult microbiome. A recent study surveyed the bacterial species in the human gut, and found these species to be most abundant [2]:
As this figure shows, Bacteroides spp. are the most common commensal bacteria, with Bacteroides uniformis alone providing almost 10% of all bacterial genes in the gut. Lactobacillus and Bifidobacterium do not appear among the 57 most abundant species.
This study showed, by the way, that patients with irritable bowel syndrome have 25% fewer types of bacterial gene in their gut than healthy people, and that the composition of bacterial genes in feces clearly distinguishes ulcerative colitis, Crohn’s disease, and healthy patients. In other words, in the bowel diseases a few pathogenic species have colonized the gut and entirely denuded it of about 25% of the commensal species that normally populate the gut. This finding supports the idea that restoring those missing species might be therapeutic for IBS.
Bacterial Replacement Therapies Work
So if IBS patients are missing 25% of the thousand or so species that should populate the gut, or 250 species, and if common probiotics provide only 8 or so species and not the ones that are missing, how are the missing species to be restored?
The answer is simple but icky. Recall that half the dry weight of stool consists of bacteria. A healthy person daily provides a sample of billions of bacteria from every one of the thousand species in his gut. They are in his stool.
So a “fecal transplant” of a healthy person’s stool into the gut of another person will replenish the missing species.
Scientists have known for a long time that this was likely to be an effective therapy, but it is only now entering clinical practice. The New York Times recently made a stir by telling this story:
In 2008, Dr. Khoruts, a gastroenterologist at the University of Minnesota, took on a patient suffering from a vicious gut infection of Clostridium difficile. She was crippled by constant diarrhea, which had left her in a wheelchair wearing diapers. Dr. Khoruts treated her with an assortment of antibiotics, but nothing could stop the bacteria. His patient was wasting away, losing 60 pounds over the course of eight months. “She was just dwindling down the drain, and she probably would have died,” Dr. Khoruts said.
Dr. Khoruts decided his patient needed a transplant. But he didn’t give her a piece of someone else’s intestines, or a stomach, or any other organ. Instead, he gave her some of her husband’s bacteria.
Dr. Khoruts mixed a small sample of her husband’s stool with saline solution and delivered it into her colon. Writing in the Journal of Clinical Gastroenterology last month, Dr. Khoruts and his colleagues reported that her diarrhea vanished in a day. Her Clostridium difficile infection disappeared as well and has not returned since.
The procedure — known as bacteriotherapy or fecal transplantation — had been carried out a few times over the past few decades. But Dr. Khoruts and his colleagues were able to do something previous doctors could not: they took a genetic survey of the bacteria in her intestines before and after the transplant.
Before the transplant, they found, her gut flora was in a desperate state. “The normal bacteria just didn’t exist in her,” said Dr. Khoruts. “She was colonized by all sorts of misfits.”
Two weeks after the transplant, the scientists analyzed the microbes again. Her husband’s microbes had taken over. “That community was able to function and cure her disease in a matter of days,” said Janet Jansson, a microbial ecologist at Lawrence Berkeley National Laboratory and a co-author of the paper. “I didn’t expect it to work. The project blew me away.” [3]
Fecal transplants can be done without a doctor’s help: someone else’s stool can be swallowed or inserted in the rectum. If taking feces orally, swallow a great deal of water afterward to help wash the bacteria through the stomach and its acid barrier.
Dogs and young children sometimes swallow feces. It is unpleasant to consider, but desperate diseases call for desperate measures. Perhaps one day, healthy stools will be available in pleasant-tasting capsules, and sold on supermarket shelves. Not yet.
Attacking Pathogenic Biofilms
Most bacterial species will build fortresses for themselves, called biofilms. These are polysaccharide and protein meshworks that, like bone, become mineralized with calcium and other minerals. These mineralized meshworks are built on bodily surfaces, like the gut lining, and protect bacteria from the immune system, antibiotics, and other bacterial species.
Pathogenic species known to generate biofilms include Legionella pneumophila, S. aureus, Listeria monocytogenes, Campylobacter spp., E. coli O157:H7, Salmonella typhimurium, Vibrio cholerae, and Helicobacter pylori. [4]
Biofilms favor the species that constructed them. So, once pathogens have constructed biofilms, it is hard for commensal species to displace them.
Therapies that dissolve pathogenic biofilms can improve the likelihood of success of probiotic and fecal transplant therapies. Strategies include enzyme supplements, chelation therapies, and avoidance of biofilm-promoting minerals like calcium. Specifically:
- Polysaccharide and protease digesting enzymes. Human digestive enzymes generally do not digest biofilm polysaccharides, but bacterial enzymes that can are available as supplements. Potentially helpful enzymes include hemicellulase, cellulase, glucoamylase, chitosanase, and beta-glucanase. Non-human protease enzymes, such as nattokinase and papain, might also help. [5]
- Chelation therapy. Since biofilms collect metals, compounds that “chelate” or bind metals will tend to gather in biofilms. Some chelators – notably EDTA – are toxic to bacteria. So EDTA supplementation tends to poison the biofilm, driving bacteria out of their fortress-shelter. This prevents them from maintaining it and makes the biofilm more vulnerable to digestion by enzymes and commensal bacteria. It also tends to reduce the population of pathogenic bacteria.
- Mineral avoidance. The supply of minerals, especially calcium, iron, and magnesium, can be a rate-limiting factor in biofilm formation. Removal of calcium can cause destruction of biofilms. [6] We recommend limiting calcium intake while bowel disease is being fought, since the body can meet its own calcium needs for an extended period by pulling from the reservoir in bone. Upon recovery, bone calcium can be replenished with supplements. Iron is another mineral which promotes biofilms and might be beneficially restricted. We do not recommend restricting magnesium.
Some commercial products are available which can help implement these strategies. For instance, Klaire Labs’ InterFase (http://www.klaire.com/images/InterFase_Update_Article.pdf) is a popular enzyme supplement which helps digest biofilms, and a version containing EDTA is available (InterFase Plus).
Attacking Biofilms With Berries, Herbs, Spices, Vinegar, and Whey
Plants manufacture a rich array of anti-microbial compounds for defense against bacteria.
There is reason to believe that traditional herbs and spices, which entered the human diet during the Paleolithic and have been passed down through the generations for tens of thousands of years, were selected by our hunter-gatherer ancestors as much for their ability to promote gut health as for their taste. Dr. Art Ayers notes that:
Plants are adept at producing a wide array of chemicals with refined abilities to block bacterial functions. So when researchers sought chemicals to solve the problem of pathogens forming biofilms, it was natural to test plant extracts for inhibiting compounds. In a recent article [7], D.A. Vattem et al. added extracts from dietary berries, herbs and spices to bacterial pathogens, including the toxin producing Escherichia coli (EC) O157:H7, and checked for the ability to produce a chemical that signals the formation of a biofilm. The effective phytochemicals inhibited the bacteria from recognizing a critical density of bacteria, i.e. quorum sensing, and responding with the production of the biofilm-triggering chemical.
Blueberry, raspberry, cranberry, blackberry and strawberry extracts were effective as quorum sensing inhibitors (QSIs). Common herbs such as oregano, basil, rosemary and thyme were also effective. Turmeric, ginger and kale were also tested and found to contain QSIs. [8]
A few other remedies can weaken biofilms:
- Acetic acid in vinegar can solubilize the calcium, iron, and magnesium in biofilms, removing these minerals and weakening the biofilm; citric acid binds calcium and can disrupt biofilms. [9]
- Lactoferrin, a molecule in milk whey, binds iron and inhibits biofilm formation and growth. [10]
- N-acetylcysteine can destroy or inhibit biofilms. [11]
Conclusion
Fecal transplants are the best probiotic. Tactics to disrupt pathogenic biofilms can assist probiotics in bringing about re-colonization of the digestive tract by commensal bacteria.
Along with a non-toxic diet (discussed in Part II) and nutritional support for the immune system and gut (discussed in Part III), these steps to improve gut flora make up a natural program for recovery from bowel disease.
UPDATE: Please read the cautions by two health professionals, annie and Jesse, about potential dangers of self-treatment with fecal transplants and EDTA. It is always better to pursue these therapies with a doctor’s assistance and monitoring.
Related Posts
Other posts in this series:
- Bowel Disorders, Part I: About Gut Disease July 14, 2010
- Bowel Disease, Part II: Healing the Gut By Eliminating Food Toxins m July 19, 2010
- Bowel Disease, Part III: Healing Through Nutrition July 22, 2010
References
[1] Lapaquette P, Darfeuille-Michaud A. Abnormalities in the Handling of Intracellular Bacteria in Crohn’s Disease. J Clin Gastroenterol. 2010 Jul 7. [Epub ahead of print]. http://pmid.us/20616747.
[2] Qin J et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010 Mar 4;464(7285):59-65. http://pmid.us/20203603.
[3] Carl Zimmer, “How Microbes Defend and Define Us,” New York Times, July 12, 2010, http://www.nytimes.com/2010/07/13/science/13micro.html.
[4] Donlan RM. Biofilms: microbial life on surfaces. Emerg Infect Dis. 2002 Sep;8(9):881-90. http://pmid.us/12194761.
[5] Tets VV et al. [Impact of exogenic proteolytic enzymes on bacteria]. Antibiot Khimioter. 2004;49(12):9-13. http://pmid.us/16050494.
[6] Kierek K, Watnick PI. The Vibrio cholerae O139 O-antigen polysaccharide is essential for Ca2+-dependent biofilm development in sea water. Proc Natl Acad Sci U S A. 2003 Nov 25;100(24):14357-62. http://pmid.us/14614140. Geesey GG et al. Influence of calcium and other cations on surface adhesion of bacteria and diatoms: a review. Biofouling 2000; 15:195–205.
[7] Vattem DA et al. Dietary phytochemicals as quorum sensing inhibitors. Fitoterapia. 2007 Jun;78(4):302-10. http://pmid.us/17499938.
[8] Art Ayers, “Spices are Antimicrobial and Inhibit Biofilms,” Dec. 7, 2008, http://herbal-properties.suite101.com/article.cfm/spices_are_antimicrobial_and_inhibit_biofilms.
[9] Art Ayers, “Cure for Inflammatory Diseases,” Sept. 2, 2009, http://coolinginflammation.blogspot.com/2009/09/cure-for-inflammatory-diseases.html. Desrosiers M et al. Methods for removing bacterial biofilms: in vitro study using clinical chronic rhinosinusitis specimens. Am J Rhinol. 2007 Sep-Oct;21(5):527-32. http://pmid.us/17883887.
[10] O’May CY et al. Iron-binding compounds impair Pseudomonas aeruginosa biofilm formation, especially under anaerobic conditions. J Med Microbiol. 2009 Jun;58(Pt 6):765-73. http://pmid.us/19429753.
[11] Cammarota G et al. Biofilm demolition and antibiotic treatment to eradicate resistant Helicobacter pylori: A clinical trial. Clin Gastroenterol Hepatol. 2010 May 14. [Epub ahead of print] http://pmid.us/20478402. Zhao T, Liu Y. N-acetylcysteine inhibit biofilms produced by Pseudomonas aeruginosa. BMC Microbiol. 2010 May 12;10:140. http://pmid.us/20462423.











Recent Comments