We have a great respect for the influence of light upon health. We’ve blogged and spoken about the importance of blue light for circadian rhythm entrainment, and ultraviolet light for production of vitamin D and nitric oxide (for example, in “Nitric Oxide and AO+Mist Skin Probiotic at the Perfect Health Retreat”; use the coupon code phd25 for 25% off AOBiome’s nitric oxide enhancing skin probiotic).
However, red and near infrared light are healthful too. Vladimir Heiskanen (also known as “Valtsu”) is a Finnish blogger and intelligent young scholar who has been researching the effects of red and near infrared light, and he wrote a post on his blog which deserves greater exposure. Vladimir has graciously allowed me to revise his post for PHD readers. – Paul Jaminet
I (Valtsu) used to spend a lot of time reading Ray Peat’s articles, trying to make sense of his ideas. In many of his articles, Peat praised red light. For example (from here):
Old observations such as Warburg’s, that visible light can restore the activity of the “respiratory pigments,” showed without doubt that visible light is biochemically active. By the 1960s, several studies had been published showing the inhibition of respiratory enzymes by blue light, and their activation by red light.
Peat didn’t give many references to justify his claims, but after doing some searches on PubMed, I realized that there are thousands of papers supporting his views.
Mechanisms
Activation of cytochrome c oxidase (Cox), the mitochondrial respiratory enzyme discovered by Nobel laureate Otto Warburg, seems to be the primary mechanism by which red light enhances mitochondrial function. [1] [2] [3] [4] [5] [6]
Cox is the centerpiece of the last stage of mitochondrial energy production, Complex IV. Cox utilizes energetic electrons and protons from opposite sides of the inner mitochondrial membrane to turn one molecule of oxygen (O2) into two molecules of water (H2O), in the process contributing the energy required to form ATP.
But a number of small molecules can displace the O2, spoiling the reaction and acting as inhibitors of ATP synthesis. These include cyanide (HCN), carbon monoxide (CO), hydrogen sulfide (H2S), and nitric oxide (NO). If too much oxygen is displaced, as in cyanide or carbon monoxide poisoning, cells die from chemical asphyxiation.
Nitric oxide is a native molecule of crucial importance for health, especially cardiovascular health. Nitric oxide generation by ultraviolet light (UV-A) is probably a major reason for the healthfulness of sunshine. Nitric oxide is commonly generated by stressed cells (for example, during the heat shock response) to support cellular health, in part by increasing blood flow.
However, the binding of NO to Cox, inhibiting mitochondrial respiration, can be an unwanted side effect. It turns out, fortunately, that red and near infrared light photodissociate NO from Cox, leading to its release from mitochondria back into circulation with beneficial effects on blood flow.
Removal of NO from mitochondria appears to be the mechanism by which red light phototherapy enhances mitochondrial respiration [2] [7a] [7b] [8] [9] [10] [11]. Supporting evidence: provision of NO abolishes the cellular effects of red and near infrared light.
Visible light doesn’t penetrate the body well, but infrared light does. Near infrared light is only reduced in intensity by about half after passage through 2 mm of tissue, and in daytime outdoor environments may dissociate NO and promote mitochondrial respiration at a depth of 2-3 cm beneath the skin. [13] [14] [15]
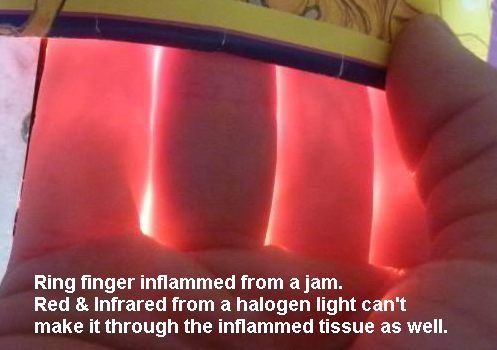
This image, from here, is illuminating:
First, it illustrates the relative transparency of human tissue in the red (and even greater transparency in the infrared). Transparency in this frequency range appears to have been evolutionarily selected, to the point that one molecule – cytochrome c oxidase in mitochondria – absorbs 35% or more of red light. If it was so important to let red light reach mitochondria that other human molecules had to evolve transparency in the red, then it is surely important for us to provide our mitochondria with red light.
Second, when tissues are injured, they release extra nitric oxide, and NO bound to Cox absorbs red light, making the injured tissue more opaque in the red. In this case the middle finger had been jammed; due to the injury it passes significantly less red light than the fingers on either side.
The History of Red Light Therapy
There is nothing new under the sun, and when a simple activity is beneficial for health, we often find that somebody discovered the effect long ago. So it is with red light.
Niels Ryberg Finsen won the Nobel Prize for Medicine in 1903 for his explorations of the therapeutic effects of light, notably the use of ultraviolet light to treat lupus and other diseases; but he had also found that red light could be beneficial, and had published an article titled “The Red Light Treatment of Smallpox” in 1895.
The idea of using red light for therapy was picked up by John Harvey Kellogg, who published a 200-page book titled Light Therapeutics in 1910. Kellogg had long been famous as one of the first vegetarian doctors, leader of the Battle Creek Sanitarium for the Seventh Day Adventist Church from 1876 until 1933, and as the inventor of corn flakes breakfast cereals (in 1878). Kellogg recommended light therapy for diabetes, obesity, chronic fatigue, insomnia, baldness, and cachexia. [18]
After the invention of lasers, it was found that red laser light could accelerate wound healing in animals, and in the 1970s similar results were obtained in humans. [2]
The most interesting work on phototherapy, however, has been conducted recently. It is becoming a hot field: a Pubmed search for LLLT (an acronym for “low-level laser therapy” or “low-level light therapy”) generates about 10 papers per year in the 1990s, 100 per year in the 2000s, and 400 per year in the 2010s.
Therapeutic Benefits from Local Application of Red Light
Therapeutic benefits from local application of red or near infrared light to injured tissues have been reported for several conditions:
- Age-related macular degeneration. The eyes of 200 elderly subjects with age-related macular degeneration were exposed to near infrared light of wavelength 780 nm. Visual acuity was improved in 95% of the subjects; most were able to see two rows lower on an eye chart. Results achieved in two weeks of treatment were maintained three to thirty-six months. [65]
- Knee osteoarthritis. Application of near infrared (830 nm) light to the knees of osteoarthritis patients dramatically reduced knee pain scores. [73]
- Herpes labialis. Cold sores around the lips caused by herpes simplex virus 1 were treated with red laser light. Time to recurrence was a median 37.5 weeks in the treatment group, 3 weeks in the placebo group. (Subjects wore masks and couldn’t tell which group they were in.) [61]
- Hypothyroidism. Hashimoto’s hypothyroidism patients were exposed to near infrared (830 nm) radiation of the skin over the thyroid gland. Nine months later, 48% of the treatment group had been able to stop taking thyroid hormone, and the average T4 dose had dropped from 93 mcg to 39 mcg. In the control group, the average T4 dose had increased from 90 mcg to 107 mcg. [37] Similar results have been reported in other studies. [36] [37] [38] [40] [41] [42] [43]
- Cognitive dysfunction following traumatic brain injury. Eleven patients with continuing cognitive dysfunction following traumatic brain injury (from motor vehicle accidents, sports injuries, and an improvised explosive device detonation) were treated with red and near-infrared light to the scalp. They experienced improvements in executive function, learning, and memory, as well as improved sleep and fewer post-traumatic stress disorder symptoms.
- Cellulite. This is more speculative, but there are indications that red and near infrared light can help reduce cellulite.
- Hair loss. Use of a laser hair comb led to fuller and thicker hair in hair loss patients.
Systemic Benefits of Phototherapy
Even when light is applied locally, some of the benefits may be shared systemically.
For instance, exposure to light causes release of NO from mitochondria and also an increase in NO levels due to photoactivation of nitric oxide synthase. Elevation of NO anywhere increases blood flow throughout the body. In one experiment, one hand was irradiated with white light; blood flow rate increased 45% in the irradiated hand and 39% in the non-irradiated hand.
Irradiation with white light has been found to increase antibody production, presumably improving immune function.
Light exposure has also been found to have anti-inflammatory effects. For example, white light exposure reduces levels of the pro-inflammatory cytokines TNF- α, IL-6, interferon-gamma, and interleukin-12 and increases levels of anti-inflammatory cytokines interleukin-10 and TGF-beta.
Other studies have found that UV radiation increases TNF-α, IL-6 and other pro-inflammatory cytokines. [90] [91] So it is likely that the anti-inflammatory effects of the white light were chiefly due to its red and near infrared components.
These anti-inflammatory effects may shed light on the improvements hypothyroid subjects experienced from near infrared phototherapy. TNF-α and IL-6 suppress peripheral thyroid hormone metabolism by decreasing T3 and increasing rT3. [92] [93] Inflammation seems to commonly trigger hypothyroidism, while anti-inflammatory strategies are almost always therapeutic for hypothyroidism.
Epidemiological evidence suggests that light exposure improves serum lipid profiles. At mid-latitudes, serum cholesterol levels typically rise 5% to 10%, but HDL cholesterol levels decrease, in winter. Blood pressure is also higher in winter. [97] [98] [99] [100] [101] [102] Lack of red light may be the reason. In a pilot study, red light exposure reduced serum cholesterol levels in 84% of subjects.
Widespread Deficiencies in Light Exposure
It’s likely that our modern environments lead to systemic deficiencies in light exposure. It’s common for health to worsen in low-light locations or seasons, as Ray Peat observed:
Many people who came to cloudy Eugene to study, and who often lived in cheap basement apartments, would develop chronic health problems within a few months. Women who had been healthy when they arrived would often develop premenstrual syndrome or arthritis or colitis during their first winter in Eugene.
Since the last ice age ended, humanity has populated more northerly latitudes and moved indoors. We are getting far less light than our ancestors. The evolutionary mismatch principle suggests that humans will be optimized for ancestral light levels, and that we moderns can improve our health by getting more light.
Health Risks of Blue-Only Light
While red light tends to enhance mitochondrial function, high intensities of blue light can damage mitochondria by triggering oxidative stress. Blue-only light can kill retinal cells. Exposing rats to blue-only light or to “white” LED light that peaks unnaturally strongly in the blue led to retinal damage. LED lights are the worst, due to their concentration at single frequencies, but compact fluorescent lights which have peaks in the blue can also generate mild to moderate retinal damage. A commentary on the research is here.
Reactive oxygen species are generated in mitochondria when they can’t dispose of electrons in the manufacture of ATP. For this reason, NO binding to Cox promotes oxidative stress, and release of NO from Cox by red and infrared light reduces oxidative stress. It is probably by this mechanism that red and near infrared light protects against retinal injury.
Because of this research, we don’t recommend using blue-only light boxes for circadian rhythm entrainment; rather, use full spectrum white light.
Incandescent lights, which produce a smooth spectrum including red and near-infrared wavelengths, are probably safest for eye health. Some researchers, such as Richard Funk and Alexander Wunsch, who appeared in the Bulb Fiction documentary, assert that increased CFL usage may be harmful to eyes. Of course, governments are here to help us, and have banned incandescent lighting.
Circadian Rhythm Considerations
There are hints that bright red and near infrared light exposure should occur in the daytime.
Night-time levels of melatonin, but not day-time levels, have been shown to abolish the effects of red and infrared light on cellular function, just as nitric oxide does.
This shows that melatonin, a circadian rhythm hormone, evolved to inhibit red light influences at night. If our “night hormone” is trying to block the influence of red light, we probably shouldn’t willfully expose ourselves to bright red light at night.
What to Do
If fluorescent lights are problematic, how should we get adequate red and near infrared light, while getting sufficient blue in the daytime to entrain circadian rhythms?
The general lighting types that provide the most red and near-infrared are incandescent lights, heat lamps, and LEDs.
Heat lamps by Philips or Osram generate little blue light but, thanks in part to their high power levels (up to 250 W), provide significant red and near-infrared light. However, only ~12% of the power is emitted at therapeutic wavelengths (600-1070 nm); most of their power is emitted at the warming IR-B wavelengths. In fact, these lamps emit so much heat that they can substitute for central heating in the winter. Most people will find they produce too much heat for summer use. Although we recommend using red or orange lighting at night, heat lamps are not really suitable for night use; ambient temperature is a zeitgeber and our exposure to warmth should occur in the daytime. Moreover, as noted above, it might be best to get red light exposure in the daytime, when melatonin levels are low. So heat lamps are best used in the daytime as a complement to other broad-spectrum lighting.
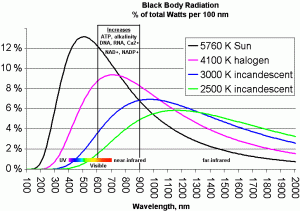
Incandescent lights (including halogen lights), which produce a blackbody spectrum, can generate a significant amount of red and near infrared and are excellent daytime light sources. A color temperature of 5500 K mimics the sun and provides substantial amounts of red and near infrared; a color temperature of 4100 K is also excellent in the red and near infrared, but is rather weak in the blue and ultraviolet for daytime circadian rhythm entrainment. A look at blackbody spectra as a function of color temperature:
LEDs are another possibility. One of their virtues is that it’s possible to obtain monochromatic LEDs with frequencies optimized for Cox-NO photodissociation (680 and 820 nm work best; inexpensive LEDs are available at 630, 660, 850, and 880 nm. Here is a video of a fellow who created a homemade LED helmet:
A variety of commercial light devices have been used in LLLT/light therapy studies: Anodyne, Bioptron, HairMax LaserComb, Omnilux, Noveon NaiLaser, Biolight, Quantum Warp, Syrolight BioBeam, HIRO 3.0, Picasso Lite, HELBO® TheraLite Laser and Mustang 2000.
Conclusion
Exposure to sunshine on bare skin, which was common in our ancestral environment, is something we need to obtain or mimic if we want optimal health.
Unfortunately, it’s hard to reproduce the many facets of sunshine in indoor environments. Blue light (for daytime circadian rhythm entrainment), UV-A light (for nitric oxide), UV-B light (for vitamin D), red light (for nighttime circadian rhythm entrainment), and now red and near infrared light in the daytime (for mitochondrial respiration) all seem to be important.
Awareness is the first step toward optimization. Thank you, Vladimir, for sorting through this research for us!













Recent Comments