I’ve had about ten requests for thoughts on the new paper in Nature Medicine [1] that finds red meat can promote atherosclerosis by a roundabout route: carnitine in the meat is metabolized by gut bacteria into a compound called TMA, which the liver converts to TMAO, which in high doses promotes growth of atherosclerotic plaques.
The same group has done similar studies with other molecules; two years ago the culprit was not carnitine but phosphatidylcholine. [2]
The Scare
Some of the news stories:
- Wall Street Journal, “New Health Worry in Red Meat”
- New York Times, “Culprit in Heart Disease Goes Beyond Meat’s Fat”
- Scientific American, “Red Meat May Clog Arteries Because of Gut Bacteria”
- ScienceDaily, “New Link Between Heart Disease and Red Meat: New Understanding of Cardiovascular Health Benefits of Vegan, Vegetarian Diets”
- The Scientist, “Steak Linked to Heart Disease”
It sounds like red meat is dangerous! The best line came from the New York Times:
Lora Hooper, an associate professor of immunology and microbiology at the University of Texas Southwestern Medical Center, who follows the Paleo diet, heavy on meat, exclaimed, “Yikes!”
The Big Picture
The issue here is closely related to one discussed in page 77 of our book:
Protein is not food for us alone; gut bacteria can ferment protein.
Although fermentation of carbohydrates by gut bacteria is usually beneficial, fermentation of protein is not: it generates toxic compounds, including amines, phenols, indoles, thiols, and hydrogen sulfide, which make a foul-smelling stool.
It seems likely, therefore, that high protein intakes are suboptimal for gut health.
When protein is fermented, nitrogen is released, and many nitrogenous compounds are toxic.
The group behind the new research, led by Stanley Hazen, has been looking at another pathway by which bacterial fermentation of meat might be dangerous – the pathway that runs through Trimethylamine. Trimethylamine (TMA) has a simple structure; three methyl groups bonded to a nitrogen atom: N(CH3)3.
Compounds such as choline and carnitine that contain both methyl groups and nitrogen are potential precursors to TMA.
TMA is responsible for the fishy smell of decaying fish. It is highly abundant in fish.
The liver converts TMA into its oxide, TMAO. The Hazen group in a series of papers has argued that higher TMAO levels in blood are associated with atherosclerosis. In a recent paper they assert, “TMAO levels explain 11% of the variation in atherosclerosis.” [3]
So, the equation they are putting together is: fermentation of meat in the gut produces TMA leading to TMAO production which may increase your chance of atherosclerosis by 11%.
Risk is Highly Dependent on the Nature of Your Gut Flora
Here is the key data from the new paper [1]. The “d3” prefix means that the carnitine was labeled with deuterium, an isotope of hydrogen, to help trace its molecular destinations.
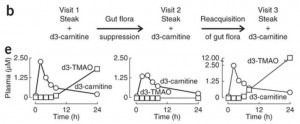 In the left panel of part (e), subjects have been fed a steak (eaten in 10 minutes) plus a 250 mg deuterated carnitine supplement – in total, the carnitine equivalent of 1.5 pounds of meat. Deuterated TMAO levels in blood rise to about 1.8 parts per million after 24 hours.
In the left panel of part (e), subjects have been fed a steak (eaten in 10 minutes) plus a 250 mg deuterated carnitine supplement – in total, the carnitine equivalent of 1.5 pounds of meat. Deuterated TMAO levels in blood rise to about 1.8 parts per million after 24 hours.
Then subjects are given antibiotics for a week to suppress their gut flora, and fed steak and deuterated carnitine again. On antibiotics, their blood has no deuterated TMAO at all 24 hours after the meal.
In the right panel, 3 weeks after coming off antibiotics to allow gut flora to regrow, subjects are challenged again with steak and deuterated carnitine. Their blood level of deuterated TMAO now exceeds 12 parts per million – 7 times higher than before the antibiotics.
They go on to test the mix of flora in subjects, and show that flora composition is closely correlated with blood levels of deuterated TMAO after consumption of deuterated carnitine. Some types of gut bacteria produce a lot of TMA from food carnitine, others produce little.
So the amount of TMAO entering the blood from bacterial metabolism of food carnitine is highly dependent on the nature of the gut flora. If you kill off normal flora with antibiotics, then eat meat and carnitine, you will get an overgrowth of bacteria specialized to feed on meat and carnitine. That might not be good for you.
The Vegan vs Omnivore Comparison
Food carnitine is far from the only source of blood TMAO. In fact, TMA is a natural breakdown product of choline, one of the most abundant molecules in the body, and the body has evolved an enzyme for converting TMA into TMAO — the gene is FMO3. So we should ask, how much does metabolism of carnitine by gut bacteria affect blood TMAO levels? For that we need measurements of normal TMAO, not just deuterated TMAO.
We can see what that data looks like in a plot comparing blood TMAO levels between vegans and omnivores (panel c of their Figure 2):
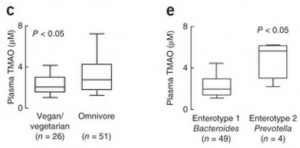 These are the TMAO levels normally circulating in the fasting blood of SAD omnivores and vegetarians. As you can see, there’s considerable overlap between the two distributions. 75% of the omnivores had TMAO levels within the same range as 90% of the vegetarians.
These are the TMAO levels normally circulating in the fasting blood of SAD omnivores and vegetarians. As you can see, there’s considerable overlap between the two distributions. 75% of the omnivores had TMAO levels within the same range as 90% of the vegetarians.
So about 75% of omnivores and 90% of vegetarians have normal TMAO levels. What about the 25% of omnivores and 10% of vegetarians whose TMAO levels are elevated?
In panel e, you can see that the enterotype of the gut flora is a much better predictor of blood TMAO levels than whether someone eats meat. Those with high Prevotella, low Bacteroides averaged about triple the TMAO levels of those with low Prevotella, high Bacteroides flora.
So it really is the gut flora that determine blood TMAO levels.
What Drives the Gut Flora?
What determines whether you have the bad gut flora?
The general picture is this. The immune system regulates the number of microbes living in the gut. When levels become high, antimicrobial peptides are released into the gut to kill some off. When levels are low, antimicrobial peptide production is reduced to let microbes multiply.
This means that if the proportion of bacteria who feed on protein, carnitine, and choline is too high, it’s probably because there is insufficient food for the competing bacteria who feed on carbohydrate forms of fiber. If you have a lot of gut bacteria feeding on fiber, there’s no room in the gut for large amounts of bacteria who feed on meat.
So the 25% of omnivores and 10% of vegan/vegetarians with high TMAO levels are probably the people on low-fiber diets – the ones who get their carbs from flour and sugar. On such a diet, the good bacteria are starved and the bad bacteria that produce TMA multiply.
How Does TMAO Produce Atherosclerosis?
The explanation offered by the Hazen group is that TMAO suppresses “reverse cholesterol transport” conceived broadly as the process of migrating excess cholesterol out of macrophages for transport to the liver and excretion in feces via the bile.
Basically, the idea here is:
- Atherosclerosis begins with metabolic syndrome, a state characterized by high LDL levels and caused by endotoxemia (high levels of endotoxins entering the body from the gut).
- As we’ve discussed (“Blood Lipids and Infectious Disease, Part II,” July 12, 2011), LDL particles have an immune function. They are oxidized by microbial cell wall components. The resulting oxLDL particles are taken up by macrophages, which then present the microbial cell wall components to other immune cells for antibody formation.
- Endotoxemia initiates the process of atherosclerosis by (a) poisoning the liver to cause metabolic syndrome which raises LDL levels, and (b) oxidizing LDL – since endotoxins are bacterial cell wall components that can oxidize LDL – and driving the oxLDL into macrophages.
- After macrophages have separated the microbial cell wall components from their accompanying LDL particle, the cholesterol and fat have to be exported to keep them from building up in the cell.
- If cholesterol and fat cannot be exported quickly enough, the macrophage is injured and becomes a “foam cell.” Disabled foam cells accumulate in specific locations and form atherosclerotic plaques.
- TMAO suppresses bile acid creation, reducing the excretion of cholesterol from the body and leading to higher LDL levels and a greater likelihood that macrophages will become foam cells.
If this is true, then TMAO is not intrinsically atherosclerotic. TMAO in blood only becomes atherosclerotic in the context of metabolic syndrome brought on by endotoxemia.
What causes endotoxemia? A dysbiotic flora generated by a diet high in sugar, flour, and omega-6 fats (see our book, pp 220-222).
Conclusion: Lessons Learned
The lessons of this study are:
- Don’t eat a high-sugar, high-flour, low-fiber diet.
- Do eat natural whole foods that have the kind of fiber we and our probiotic gut flora co-evolved eating; mainly, resistant starch from in-ground starches like potatoes and soluble fiber from fruits and vegetables.
- Don’t eat excessive amounts of meat. As we noted in the book, excess protein is available to gut bacteria for fermentation and that produces a number of toxic byproducts.
- Do eat PHD levels of meat – one-half to one pound per day. This level of meat consumption will provide healthful and nourishing amounts of protein, choline, and carnitine, and will not cause any harm if accompanied by PHD levels of healthy plant foods.
None of these lessons is new. This study doesn’t overturn any established dietary wisdom. It is just one more piece of data reminding us to eat a balanced diet consisting of the foods we evolved eating – plant as well as animal.
References
[1] Koeth RA et al. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013 Apr 7. http://pmid.us/23563705.
[2] Wang Z et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011 Apr 7;472(7341):57-63. http://pmid.us/21475195.
[3] Bennett BJ et al. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab. 2013 Jan 8;17(1):49-60. http://pmid.us/23312283.











Recent Comments