Matt Farina is in the process of using the Perfect Health Diet to recover from hypothyroidism, and wanted to report his progress to PHD readers. Here’s Matt! – Paul Jaminet
My hypothyroidism was discovered in 2012. I was age 30 at the time and had just moved to a new part of the UK and enlisted with a new doctors surgery. The condition was discovered by accident when a nurse at the new surgery noticed I had an irregular heart beat. I was sent for blood tests in April 2012 where it was discovered that I had an underactive thyroid. My serum TSH was 11.3. We retested a week later and it was similar, 11.7. Free T4 was 11.1 and 11.6 pmol/l, on the low side.
The doctor asked if I had any of the common symptoms of hypothyroidism; I said I was tired in the day and had brain fog in the morning. I regularly go to the gym and run 10k and half marathon races, so the tiredness was noticeable. My mood was sometimes a little unstable too.
The doctor prescribed 50 mcg levothyroxine, and I began taking it daily. In July 2012, we remeasured. With the levothyroxine, my TSH had fallen to 3.0 and my free T4 had risen to 17.6 pmol/L – right in the middle of the normal range. Nevertheless, my hypothyroidism symptoms, such as brain fog and tiredness, persisted.
In early 2013, I relocated to a new part of the country and found a new doctor. They remeasured and my TSH was now 2.71. In July 2013, it was 2.6. I was still taking 50 mcg levothyroxine daily.
In early 2014, I moved again, and found a new doctor. My TSH had risen a little, to 3.3.
I decided to start researching causes and cures of hypothyroidism, and started to listen to podcasts from Ben Greenfield and Robb Wolf. Through them, I noticed that some people were successfully curing hypothyroidism through the Paleo diet. This really piqued my interest and I wanted to know more.
In May 2014, I sent Ben Greenfield an email saying I was considering starting a Paleo diet to overcome my hypothyroidism and I really wasn’t expecting a reply considering how busy he must be. He kindly responded very quickly saying, “I would say Paleo may be a bit dangerous, ideally I encourage you to follow a diet closer to this one.” He linked to the Perfect Health Diet.
A new set of blood tests were taken a few days after Ben’s email which showed a significant decrease in serum free T4 and an increase in TSH levels; free T4 was down to 12.1 and TSH was up to 6.42.
The doctor wanted to double my daily dose of levothyroxine to 100 mcg per day. I asked him why he thought I had hypothyroidism, and whether he would recommend that I change my diet to help overcome it. His responses shocked me. He said my hypothyroidism “could be genetic” and “unfortunately it’s just who you are.” He also said that he wouldn’t recommend any dietary changes because I didn’t show symptoms of food intolerance.
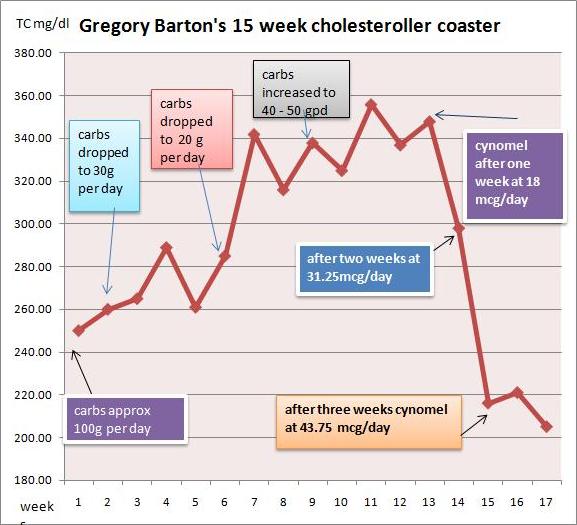
I decided that I would stick with the 50 mcg dose of levothyroxine, and see if diet could help me. I started the Perfect Health Diet the next day – June 2, 2014.
The first 3 months on the Perfect Health Diet yielded weight loss and other health benefits. I was never over weight but I dropped a jean size, started noticing stomach muscles and people were commenting on me losing weight in my face. As I intermittently fasted in the mornings, my clarity of thinking improved at work in the mornings with reduced brain fog. Other benefits included easier breathing through my nose, reduced tiredness in the day, and no more heart palpitations. The first few weeks were a little tough as my body was becoming fat adapted but that soon passed. The diet left me feeling satiated the vast majority of the time and the food I was having were truly delicious combinations. I used the Perfect Health Diet audiobook to scrub up on knowledge while I was at the gym.
After 3 months on the Perfect Health Diet, in late August 2014, I had a set of blood tests. My serum free T4 was back to normal at 18.0 and my TSH had dropped to 5.25. Nevertheless, my doctor still wanted to double my levothyroxine dose to 100 mcg. I politely declined as I knew the diet was working and that there would be even better results to come.
After 6 months on the Perfect Health Diet, in November 2014, free T4 was up again to 19.2 pmol/L and TSH had dropped again to 3.78. At this point the doctor had no comment except to say that I should stay at 50 mcg of levothyroxine daily.
At my next test, in March 2015, free T4 was up again to 19.9 pmol/L and TSH had dropped to 2.08. I continued to take 50 mcg levothyroxine daily, though in retrospect this would have been a good time to begin dropping the dose.
At my 12 month anniversary of adopting the Perfect Health Diet, in June 2015, I was tested again. This time free T4 was 27.6 pmol/L – well above normal – and TSH was only 0.12. I was now overdosing on thyroid hormone.
The doctor suggested cutting my levothyroxine dose in half, to 25 mcg per day, but I’ve decided to drop levothyroxine entirely and see if I show hypothyroidism symptoms. If I do I’ll try small doses of levothyroxine to see what works for me. I’m eagerly looking forward to my next blood test in three months to see if I am back to normal.
I fully intend to continue the Perfect Health Diet indefinitely, and I hope my story helps other people overcome their hypothyroidism.
Back in May 2014, when I first learned about PHD and the hope that it gave for natural thyroid healing, and heard my doctor say that hypothyroidism was genetic and incurable and that I would be living with it for the rest of my life, I left the doctors office feeling sad. If diet could heal hypothyroidism, then many people were being misled by their GPs.
Now I feel hope. I know hypothyroidism can be healed. Thanks for you hard work Paul, it’s really appreciated.












Recent Comments