Hippocrates famously said, “All disease begins in the gut.” I think we can narrow it down further: much modern disease begins in the mucus.
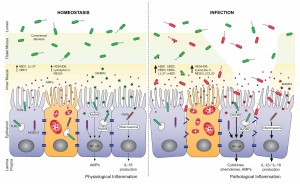
In a recent post, I put up this picture:
It’s from a paper on the role of antimicrobial peptides in maintaining gut health. [1] The point of the left panel is that a healthy gut is characterized by a thick mucosal layer that shields our intestinal and immune cells from direct contact with bacteria. The inner mucus layer is infused with antimicrobial peptides to minimize its bacterial content. The outer mucus layer contains a population of friendly mucin-degrading bacteria – symbionts like Akkermansia who evolved to feed on our mucus. These friendly bacteria provide another layer of defense against infectious pathogens; bacteria tend to be quite good at keeping out competitors. Akkermansia has been found to prevent obesity.
In an unhealthy gut, on the other hand, the mucosal layer often gets stripped away. The image (right panel) attributes this to infection, which is one possibility, but nutritional factors also matter. For example:
- Deficiencies of vitamins A or D will reduce production of antimicrobial peptides, making it easier for pathogens to reach our gut cells;
- On very low-carb diets, production of mucin-2, the primary constituent of gut mucus, may be limited in order to preserve glucose for the brain (see “Dangers of Zero-Carb Diets, II: Mucus Deficiency and Gastrointestinal Cancers,” Nov 15, 2010);
- Deficiencies of dietary fiber, vinegar, choline, and other nutrients may impair gut motility, leading to concentrations of partially digested food and bacteria at specific points in the intestine.
Regardless of why it happens, once bacteria come into direct contact with our gut and immune cells, they trigger inflammation and tend to loosen the gut barrier. This allows live bacteria and cell wall components from dead bacteria to enter the body from the gut.
Endotoxins – toxic compounds released when bacteria die, such as lipopolysaccharides from the cell walls of gram-negative bacteria – are immunogenic and inflammatory. A large influx of endotoxins into the body is “endotoxemia” – poisoning by endotoxins. As little as 2 nanograms LPS per kilogram body weight will induce fever, and 1 microgram LPS per kilogram of body weight will induce shock. [2]
Diseases caused by endotoxemia include:
- Hepatitis [3]
- Diabetes [4]
- Heart disease [5]
- Obesity [6]
- Pulmonary hypertension [7]
- Dyslipidemia [8]
- Chronic kidney disease [9]
Many of today’s most prevalent diseases are caused by chronic endotoxemia.
So it behooves us to avoid it. If endotoxemia is fundamentally caused by the loss of a protective mucosal layer in the gut, how do we assure healthy production of mucus?
A recent paper sheds valuable light on that question.
Natural Whole Foods, High-Fat Diets, and Gut Health
It’s well known that in mice, “high-fat diets” induce endotoxemia. But these diets aren’t necessarily high in fat – any pelleted rodent food in which fat provides more than 20% of calories may be called “high-fat.” The critical difference of “high-fat diets” from chow is that they are composed of purified nutrients – starch, sugar, oil, vitamins, and minerals – whereas chow is composed of natural whole foods such as wheat, corn, and seeds.
A recent study tried to distinguish whether the cause of endotoxemia is the fat, or the purified starch, sugar, and oil. It made up three diets of varying fat content (8%, 48%, and 74% of energy respectively), but composed of natural whole foods. [10]
The result was remarkable:
[U]sing complex [i.e. natural whole foods] HFD, no associations were observed between dietary lipid amounts and the magnitude of endotoxemia, inflammation, and physiological alterations developed.
It turns out the endotoxemia that typically develops on high-fat diets is due to getting the calories from purified sources – starch, sugar, oil – rather than from whole foods. On a whole foods diet, the amount of endotoxin entering the body is more or less independent of the amount of fat consumed.
This is surprising in one respect. Lipopolysaccharide is fat-loving and enters the body along with dietary fat. So it stands to reason that a higher-fat diet would carry more endotoxin into the body.
But it turns out the body has mechanisms to regulate how much endotoxin enters the body. It wants a small amount so that the immune system can sample the gut microbiome, but not so much as to cause inflammation.
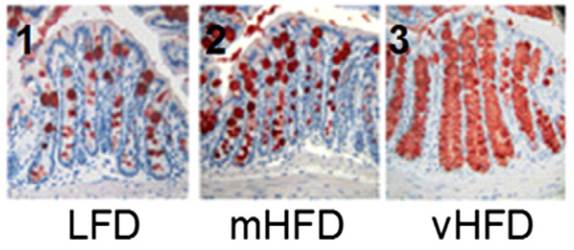
The primary mechanism for controlling endotoxin influx? Mucus production. The study noted that the mice eating higher-fat “had an increased number of goblet cells … [and] an increased MUC2 production.” MUC2 is mucin-2, the primary component of mucus in the gut.
Here is a picture with mucin-2 in the mucin-producing goblet cells stained red:
It’s obvious that mucin production goes up dramatically as the fat content of the diet increases.
The study concludes,
“We show that, in complex HFDs based on chow ingredients and milk fat, there was no association between dietary lipid amounts and the magnitude of metabolic endotoxemia or low-grade inflammation.”
If high-fat diets are healthy, we can thank our mucin-producing goblet cells.
One last note: the fact that mice can produce healthy amounts of mucus on a 74% fat diet does not necessarily mean that humans can do the same. Humans have much larger brains than mice, and as a result our carbohydrate needs are larger. It’s possible that mice can maintain mucus production on a low-carb diet better than humans can.
References
[1] Muniz LR, Knosp C, Yeretssian G. Intestinal antimicrobial peptides during homeostasis, infection, and disease. Front Immunol. 2012 Oct 9;3:310. http://pmid.us/23087688.
[2] Warren HS et al. Resilience to bacterial infection: difference between species could be due to proteins in serum. J Infect Dis. 2010 Jan 15;201(2):223-32. http://pmid.us/20001600.
[3] Parlesak A, Schäfer C, Schütz T, Bode JC, Bode C. Increased intestinal permeability to macromolecules and endotoxemia in patients with chronic alcohol abuse in different stages of alcohol-induced liver disease. J Hepatol. 2000 May;32(5):742-7. http://pmid.us/10845660.
[4] Moreno-Navarrete JM et al. Circulating lipopolysaccharide-binding protein (LBP) as a marker of obesity-related insulin resistance. Int J Obes (Lond). 2012 Nov;36(11):1442-9. http://pmid.us/22184060.
[5] Lepper PM et al. Association of lipopolysaccharide-binding protein and coronary artery disease in men. J Am Coll Cardiol. 2007 Jul 3;50(1):25-31. http://pmid.us/17601541.
[6] Cani PD et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007 Jul;56(7):1761-72. http://pmid.us/17456850.
[7] Dschietzig T, Alexiou K, Richter C, Bobzin M, Baumann G, Stangl K, Brunner F. Endotoxin causes pulmonary hypertension by upregulating smooth muscle endothelin type-B receptors: role of aldose reductase. Shock. 2008 Aug;30(2):189-96. http://pmid.us/18091567.
[8] Lassenius MI et al. Bacterial endotoxin activity in human serum is associated with dyslipidemia, insulin resistance, obesity, and chronic inflammation. Diabetes Care. 2011 Aug;34(8):1809-15. http://pmid.us/21636801.
[9] McIntyre CW et al. Circulating endotoxemia: a novel factor in systemic inflammation and cardiovascular disease in chronic kidney disease. Clin J Am Soc Nephrol. 2011 Jan;6(1):133-41. http://pmid.us/20876680.
[10] Benoit B et al. Increasing fat content from 20 to 45 wt% in a complex diet induces lower endotoxemia in parallel with an increased number of intestinal goblet cells in mice. Nutr Res. 2015 Apr;35(4):346-56. http://pmid.us/25687164.













Recent Comments