In a comment to my post “Vitamin D Dysregulation in Chronic Infectious Diseases,” Charles Colenaty, who is in his 80s, reports that high doses of vitamin D, assisted by curcumin, have cured his high blood pressure, age-related macular degeneration, bone and tooth decay, enlarged prostate, and graying hair:
Stumbled upon your site while searching for information abouit vitamin d dysreguiation and was so impressed that I had to tell you so. You gave me a much more comprehensive insight into some of vitamin D’s ecosystem that I had never imagined might be the case.
All of which prompts me to mention my vitamin D enigma that has my doctor stumped. When I retired 15 years ago as a consulting psychologist I moved from the San Francisco Bay area to the Seattle area to be close to my son. Then I got so caught up in using the computer to follow a range of interests that I seldom got out of doors — and the latitude here limits the D I could get from sunlight anyway — and I get virtually no vitamin D from my diet since I am allergic to seafood. The upshot was that as I moved into my 80’s I was confronted with a variety of physical changes that that I now think were due to severe vitamin D deficiency. Three or four teeth just crumbled over a period of a month or so, I developed adult scoliosis, and my blood pressure (always a bit high) went out of control, hitting the 190’s and low 200’s. I refused blood pressure pills since I had previously been damaged by them, and instead began taking increasing amounts of vitamin D. When I hit 15,000 a day it began to drop, and settled at the 150 to 175 range. Three months ago my vitamin D level was measured as part of a yearly physical exam, and when my doctor found that my NgL level was 92 he said that he had never seen one that high and asked me to cut my intake to 10,000 units for starters. I had tried to do that three times previously, and my blood pressure went back up the first two times and the third time my face began to swell. This fourth time didn’t work either, with my blood pressure going up after a few days of starting. I stuck it out for two weeks and then went back to the 15,000 IUs. But, as opposed to my three earlier tries, when blood pressure was back to my normal in a week, this time it took a six weeks before the blood pressure came down again. So the enigma that I have has to do with this weird relationship between my vitamin D “requirement” and my blood pressure.
Otherwise I feel better than fine. My Google research led me to a curcumin program a while back and that has brought back my original dark brown hair color, and recently I found that I now longer had to get up to go to the bathroom every night(as has been the case for years). And as of a week ago I found that my prostate is shrinking. More importantly, the AMD I have in both eyes is gradually reversing to the point where I no longer am a member of the enlarged print gang. So as far as I know everything is working fine and I don’t have a chronic anything!
These conditions rarely regress on conventional medical treatments, so to achieve this degree of success is a medical miracle.
Like Charles’s doctor, I was stumped by this at first, but then I thought to look up some other case reports of patients who benefited from super-normal 25OHD. Autism reports from Dr. John Cannell of the Vitamin D Council gave me an idea that might solve Charles’s enigma.
Background on Vitamin D
For most people, health is optimized by obtaining about 4,000 IU/day of vitamin D3 from sun or supplements, leading to a serum 25-hydroxyvitamin D (25OHD) level of 35 to 50 ng/ml in people of Eurasian ancestry or 30 to 40 ng/ml in people of African ancestry.
Not long ago I did a post on the characteristic pattern of vitamin D dysregulation in chronic infections. In chronic infectious diseases, low 25OHD is often found with elevated levels of the more active metabolite 1,25-dihydroxyvitamin D (1,25D). Possible mechanisms for this include:
- Infections making cell membranes leaky to 1,25D, causing it to spill out of cells into the blood, thus reducing activation of the nuclear membrane’s vitamin D receptor (VDR).
- Infections obstructing or downregulating the VDR, causing the body to attempt to upregulate VDR activation by increasing conversion of 25OHD to 1,25D. Both forms of vitamin D are active ligands for the VDR, but 1,25D is far more active, so converting 25OHD to 1,25D means more activation of the VDR.
Inventing ways to block the VDR or move 1,25D out of the cell would be fitness-enhancing mutations for bacteria or viruses, since activation of the VDR triggers production of antimicrobial peptides that are central to intracellular immunity. Since bacteria evolve a lot faster than humans, it should be no surprise that pathogens have been able to evolve these capabilities.
But Some Diseases Have The Opposite Pattern
But some people have diseases that produce the opposite pattern. In their diseases, “normal” 25OHD levels are associated with impaired health, while unnaturally high 25OHD levels normalize health.
Charles is a great example:
- He is taking super-normal amounts of vitamin D: Sunshine alone will generally not produce sustained creation of more than 4,000 IU/day. (Yes, I know that 10,000 IU can be produced in half an hour in D-deprived individuals, but if that person went out in the sun every day vitamin D production would soon decrease.) So 15,000 IU/day is roughly four times the normal dose.
- He is achieving super-normal levels of 25OHD that would probably be toxic for most adults. The maximum 25OHD levels achievable through sunshine vary among persons, but are generally between 48 and 80 ng/ml. [1] Moreover, human cells turn on the gene CYP24A1, which codes for the main vitamin D-degrading enzyme, at 25(OH)D levels below 100 ng/ml. [2] It seems that evolution has designed us to keep 25OHD levels around 50 ng/ml or lower – certainly below 80 ng/ml. So Charles’s 92 ng/ml is well above the levels achievable by natural methods.
Since both 25(OH)D production and degradation have been strongly selected for by evolution, we can be confident that in healthy people of reproductive age it’s not a good idea to supplement at 15,000 IU/day or drive serum 25(OH)D to 92 ng/ml.
And what limited clinical evidence we have supports that conclusion. Those tropical lifeguards who get their serum 25(OH)D levels up to 80 ng/ml? They have three times the rate of heart attacks of those with normal 25(OH)D. [3]
Aside: Their high rate of heart disease may be due to vitamin K2 deficiency. Charles, please be sure to supplement vitamin K2, preferably a mix of MK-4 and MK-7 forms, along with your D.
Yet whereas healthy younger people would experience toxicity at Charles’s vitamin D dose or 25OHD level, Charles’s health improves.
Autism and Vitamin D
Let’s consider a few other cases where super-physiological 25OHD levels have cured diseases. Dr. John Cannell of the Vitamin D Council is the most prolific writer on the subject of vitamin D, and in his newsletter has collected a number of reports of diseases being cured by pharmacologic doses of vitamin D.
Here’s a sample case report of the recovery of an autistic child, from the January 2010 newsletter. My comments are italicized within brackets:
At age 2.5 years, between December 2007 and January 2008, my son experienced a fairly dramatic onset of symptoms that led to his diagnosis of autism….
Neither the DAN Doctor nor our pediatrician would write a prescription for a therapy light, so we purchased one on our own and found it made no discernible impact on his symptoms. [PJ: No matter how much sunlight or UV light the child is exposed to, it is not possible to raise 25OHD levels enough to impact the disease.]…
I … decided we would try a vitamin D supplement. Our pediatrician did not encourage any dose higher than 400 i.u. (that found in a typical multivitamin) but did write a script to have his 25-hydroxy level tested. In August his level was 37, so we started him on 5,000 iu daily [PJ: Since vitamin D needs scale by body weight and this is a young child, this is a very high dose – comparable to Charles’s 15,000 IU] and had his level retested on October 21st. By October his level was 96 ng/ml [PJ: A super-normal level, close to Charles’s 92 ng/ml] The pediatrician was concerned that this was too high and told us he should not have more than 400 iu per day.
Knowing that Nov–March are typically his worst months, we reduced the dosage down only to 3,000 iu from October through mid-December. At an appointment in December our son was doing wonderfully (none of his usual fall/winter symptoms yet evident) and the pediatrician told us 3,000 iu was too much and that we should be giving no more than 400 iu. In mid-December we reduced the dose to 1,500 iu. [PJ: This would still be a high dose for a normal 4 year old] By the beginning of January we noted a marked loss of eye contact. [PJ: But this “high” dose is insufficient] We also noted that our son was again interchanging his right hand for writing and eating (after using his left hand exclusively for 8+ months). We increased his vitamin D level to 4,000 iu daily in early January. On January 11 we had his 25-Hydroxy level checked on January 11 and found that it was 89. [PJ: Again, the disease is present at a “normal” 25OHD of 37 ng/ml but absent at a super-normal level around 90 ng/ml.] By the end of January, we and his grandparents noted improvement in his eye contact.
In January 2010 we attended his preschool conferences. The teacher had marked cards with the following code (1=age appropriate, 2=developing, 3=area of concern). Our son received 1s in all areas with the exception of hopping on one foot and balance beam where he received 2s. We were told that he is on par with or ahead of his peers in all areas (academic, fine motor, etc.), and that his teacher had noted no unusual symptoms or concerns.
So the child’s autism is essentially cured on super-normal doses of vitamin D that raise serum 25OHD to around 90 ng/ml.
Is it just a coincidence that Charles and the autistic child experienced a normalization of health at the same 25OHD level? And that in both cases, the normalization occurs after a few weeks of high-dose vitamin D supplementation?
Hypothesis: Impaired Production of 1,25D from 25OHD
Let’s step back for a moment and think about what would cause health to normalize with super-normal 25OHD.
Suppose that for some reason, cells were unable to convert 25OHD to 1,25D. What would happen?
First, cells would have unusually low levels of 1,25D for any given level of 25OHD. Since 1,25D is more than a hundred-fold more active as a VDR ligand than 25OHD, this means that their level of VDR activation would be reduced.
By how much? In many cells, there seems to be a nearly equal balance between 25OHD and 1,25D activation of the VDR. As one paper notes:
the high serum concentration of 25(OH)D3 [500–1000 times higher than 1,25(OH)2D3] overcomes its low affinity for the receptor [500 times lower than 1,25(OH)2D3]. [4]
If the higher activity of 1,25D is almost precisely balanced by its lower abundance, then a cell’s loss of ability to make 1,25D will cut VDR activation in half.
So to restore VDR activation to normal levels, you would need to raise 25OHD to double normal levels: 70 to 100 ng/ml.
This would fit the cases of the autistic child and of Charles, both of whom reached normal health at around 90 ng/ml.
Genetic Defects: Pseudo-Vitamin D Deficiency Rickets
Mutations in the gene CYP27B1, which codes for the enzyme that turns 25(OH)D into 1,25D, create a disease called pseudo-vitamin D deficiency rickets (PDDR) or vitamin D-dependent rickets type I (VDDR I). [5]
PDDR is characterized by muscle weakness and rickets.
One nice thing about diseases caused by a single genetic defect is that they are easily reproduced in animals. PDDR can be reproduced in mice by knocking out the CYP27B1 gene.
CYP27B1 knockout mice are growth retarded, hypocalcemic, and have poor bone mineralization. The negative effects are all apparent at normal 25OHD levels of 36 ng/ml. But when the mice were given high doses of vitamin D, raising 25OHD levels to 144 ng/ml, their health was normalized. [4]
Other insights into inadequate 1,25D production have been obtained through mice deficient in vitamin D receptors. Their characteristics:
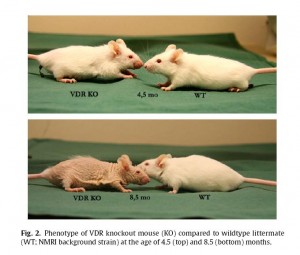
VDR mutant mice have growth retardation, osteoporosis, kyphosis, skin thickening and wrinkling, alopecia, ectopic calcification, progressive loss of hearing and balance as well as short lifespan. [6]
“Alopecia” is hair loss. “Kyphosis” is the familiar hunchback that many elderly develop. Osteoporosis is a familiar symptom of aging, as is loss of muscle, wrinkled skin, hardening of the arteries and stiffening of joints (“ectopic calcification”), loss of hearing and balance, and – approaching death.
These are all symptoms of a syndrome that is commonly called “aging.”
Here is what VDR knockout mice look like [7] (click to enlarge):
Note what happens when you can’t activate the VDR: hair loss, wrinkled skin. You get old before your time. VDR knockout mice die at an age of 10.6 months, compared to 20.5 months in wild-type mice. [7]
Our Cases Resemble PDDR
In his essay “Vitamin D Theory of Autism,” (http://www.vitamindcouncil.org/health/autism/vit-D-connection.shtml) Dr. Cannell notes similarities between PDDR and autism:
While no one has assessed afflicted [with PDDR] children for signs of autism, these children clearly display autistic markers such as hypotonia (flabby muscles), decreased activity, developmental motor delay, listlessness, and failure to thrive.
It is quite possible that autism results, as Dr. Cannell argues, from insufficient activation of the VDR during developmental ages. [8]
Similarly, what about the conditions Charles suffered from? Tooth loss, bone mineral deficiencies, and scoliosis are all classic manifestations of rickets, and vitamin D deficiency is a known risk factor for high blood pressure and for arterial hardening. Finally, his recovery of hair color might be a result of restored vitamin D function: the VDR promotes hair cycling. [9]
What Mechanisms Might Produce a CYP27B1 Deficiency in the Elderly?
It’s a safe bet that Charles does not have a genetic defect in CYP27B1. If he has a CYP27B1 dysfunction, it must have been acquired in old age.
What could have created the problem? I don’t know, but speculation is permitted at PerfectHealthDiet.com. Two possibilities are:
- Infection with a pathogen that interferes with CYP27B1. Pathogens have evolved ways to interfere with other human proteins in order to suppress the immune response. Since CYP27B1 creates 1,25D which enhances immunity, it would not be a surprise if some pathogen had evolved a way to interfere with CYP27B1.
- Mitochondrial dysfunction. The enzyme coded by CYP27B1 operates in the inner mitochondrial membrane. Only in mitochondria can 1,25D be created. The “mitochondrial theory of aging” holds that mitochondrial decay is the primary cause of aging. Perhaps in elderly people suffering from mitochondrial dysfunction, CYP27B1 does not operate properly.
Conclusion
Whatever the mechanism of CYP27B1 loss-of-function may be, it appears that doubling 25OHD levels remedies much of the loss-of-function within a few weeks.
It might not be amiss for elderly patients and autistic children with symptoms of vitamin D deficiency to experiment with raising 25OHD to twice normal levels. In those with a CYP27B1 defect, this may produce an amazing recovery.
Further recovery might be possible. If the cause is infectious, appropriate antibiotics could help. If the cause is mitochondrial decay, then mitochondrial supplements might help.
The centrality of vitamin D function to optimal aging raises another thought. What if the main cause of aging is not the decay of mitochondria in general, but a specific decay in their support for 1,25D formation in the mitochondrial inner membrane? What if this loss of intracellular 1,25D is widespread among the elderly?
In that case, following Charles’s protocol and raising 25OHD in the elderly might significantly extend lifespans. And improve hair and skin at the same time!
Related Posts
“Vitamin D Dysregulation in Chronic Infectious Diseases,” https://perfecthealthdiet.com/?p=421, August 21, 2010.
References
[1] Heaney RP. Vitamin D in health and disease. Clin J Am Soc Nephrol. 2008 Sep;3(5):1535-41. http://pmid.us/18525006.
[2] Lou YR et al. 25-Hydroxyvitamin D(3) is an agonistic vitamin D receptor ligand. J Steroid Biochem Mol Biol. 2010 Feb 15;118(3):162-70. http://pmid.us/19944755.
[3] Rajasree S et al. Serum 25-hydroxyvitamin D3 levels are elevated in South Indian patients with ischemic heart disease. Eur J Epidemiol. 2001;17(6):567-71. http://pmid.us/11949730.
[4] Rowling MJ et al. High dietary vitamin D prevents hypocalcemia and osteomalacia in CYP27B1 knockout mice. J Nutr. 2007 Dec;137(12):2608-15. http://pmid.us/18029472.
[5] Takeda E et al. Vitamin D-dependent rickets type I and type II. Acta Paediatr Jpn. 1997 Aug;39(4):508-13. http://pmid.us/9316302.
[6] Tuohimaa P. Vitamin D and aging. J Steroid Biochem Mol Biol. 2009 Mar;114(1-2):78-84. http://pmid.us/19444937.
[7] Keisala et al. Premature aging in vitamin D receptor mutant mice. J Steroid Biochem Mol Biol. 2009 Jul;115(3-5):91-7. http://pmid.us/19500727.
[8] Cannell JJ. On the aetiology of autism. Acta Paediatr. 2010 Aug;99(8):1128-30. http://pmid.us/20491697. Cannell JJ. Autism and vitamin D. Med Hypotheses. 2008;70(4):750-9. http://pmid.us/17920208.
[9] Haussler MR et al. The nuclear vitamin D receptor controls the expression of genes encoding factors which feed the “Fountain of Youth” to mediate healthful aging. J Steroid Biochem Mol Biol. 2010 Jul;121(1-2):88-97. http://pmid.us/20227497.











Recent Comments