Earlier this week a paper was released to much fanfare, claiming that diets with over 20% of energy as animal protein might be as life-threatening as smoking.
- The Huffington Post said, “Atkins aficionados, Paleo enthusiasts, and Dukan devotees, you may want to reconsider what’s on your plate. While high-protein diets have been all the rage over the last few years for their waist-whittling goodness, a new study says they could be as bad for you as smoking.”
- Scientific American said “People who eat a high-protein diet during middle age are more likely to die of cancer than those who eat less protein, a new study finds.”
- NPR said, “Americans who ate a diet rich in animal protein during middle age were significantly more likely to die from cancer and other causes.” They added, “In an age when advocates of the Paleo Diet and other low-carb eating plans such as Atkins talk up the virtues of protein because of its satiating effects, expect plenty of people to be skeptical of the new findings.” A sound prognostication!
Ray, Alex, Navy87Guy, Kat, Sam, and others asked for my thoughts.
What the Researchers Did
The article appeared in Cell Metabolism, a high-impact journal which likes long complex papers reporting years of work. [1] A common strategy for getting into such journals is to piece together a great variety of work into one article, weaving a narrative theme to unite them. That’s what this article did, using the theme “high protein diets may shorten lifespan” to link several relatively disconnected projects.
The NHANES Findings
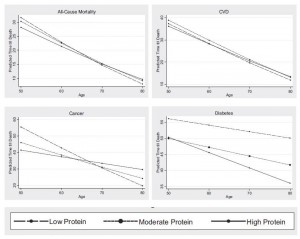
The work that generated most of the buzz was an analysis of data from the National Health and Nutrition Examination Survey (NHANES). They looked at a group of 6,381 NHANES respondents and found, “Respondents aged 50–65 reporting high protein intake had a 75% increase in overall mortality and a 4-fold increase in cancer death risk during the following 18 years. These associations were either abolished or attenuated if the proteins were plant derived.”
Here’s their Figure 1:
Two oddities in this result raise red flags:
- First, protein appears harmful at age 50, neutral at age 65, and beneficial at age 80. This reversal of effects is incompatible with most mechanisms by which protein could affect aging or disease risk. In animal studies, we see the opposite: protein restriction extends maximum lifespan, which means that at high ages, mortality is lower, but increases risk of early death, which means that in middle age mortality is higher.
- Second, they report that the effect was specific to animal protein: “[W]hen the percent calories from animal protein was controlled for, the association between total protein and all-cause or cancer mortality was eliminated or significantly reduced, respectively, suggesting animal proteins are responsible for a significant portion of these relationships. When we controlled for the effect of plant-based protein, there was no change in the association between protein intake and mortality, indicating that high levels of animal proteins promote mortality.” Yet, plant and animal proteins are biologically similar.
These two oddities strongly suggest that the appearance of negative health outcomes from protein is due to confounding factors – behaviors or foods associated with animal protein consumption in middle age, rather than effects caused by the protein itself.
When we look at how the analysis was performed, we find more reasons to doubt that protein is at fault. All of this data was found using a model which adjusted for the following covariates:
Model 1 (baseline model): Adjusted for age, sex, race/ethnicity, education, waist circumference, smoking, chronic conditions (diabetes, cancer, myocardial infarction), trying to lose weight in the last year, diet changed in the last year, reported intake representative of typical diet, and total calories.
Adjustment for a host of health-related conditions – waist circumference, diabetes, cancer, myocardian infarction, and even total calories which is effectively a proxy for obesity – can radically distort results, and even transform effects from positive to negative. I’ve discussed this issue previously in The Case of the Killer Vitamins.
In practice, many factors are highly correlated. The variables being studied – protein intake, waist circumference, total calorie intake, and others – are beset by the problem of collinearity. Attempting multiple regression analysis on collinear variables can generate very peculiar results. The more the number of adjustment factors grows, the more strange things tend to happen to data.
If they wanted us to understand whether their results are trustworthy, authors would present raw data, and then a sensitivity analysis that shows how introducing each covariate individually affects the results, then showing how including combinations of two covariates affects the results, and so forth. This would help us judge how robust the results are to alternative methods of analysis.
Of course, authors do not do this. Instead, they ask us to trust the analysis they have chosen to present – which is only one of billions they could have done. (This study adjusted for 13 covariates. The NHANES survey may have gathered data on, say, 40 variables. There are 40 choose 13, or 12 billion, possible multivariate regression analyses that could be performed using 13 covariates on this data set. Each of the 12 billion analyses would generate different outcomes.)
Are the authors trustworthy? Unfortunately, most academics today are not. Career and funding pressures are severe, and by and large those who are good at gaming the funding and publishing processes have triumphed professionally over careful, diligent truth seekers. It is much easier to construct a narrative that will garner attention and publicity and interest, than to carefully exclude non-robust results and publish only those results that are solidly supported.
Frankly, I give little credence to their NHANES analysis. And, judging by comments in the press, other epidemiologists don’t seem to give it much credence either. From the NPR article:
But could eating meat and cheese really be as bad for you as smoking, as the university news release describing the new Cell Metabolism paper suggested?
Well, that may be an exaggeration, according to Dr. Frank Hu, a researcher at the Harvard School of Public Health who studies the links between health, diet and lifestyle.
“The harmful effects of smoking on cancer and mortality are well-established to be substantial, while the harmful effects of red meat consumption are modest in comparison,” Hu wrote to us in an email.
The Mouse Experiments
So let’s turn to the next part of the study, the mouse experiments:
Eighteen-week-old male C57BL/6 mice were fed continuously for 39 days with experimental, isocaloric diets designed to provide either a high (18%) or a low (4%–7%) amount of calories derived from protein …
The low protein diets are really starvation diets, in terms of protein intake. The reason the low protein diets were sometimes 4% and sometimes 7% was because mice will often lose weight on 4% protein diets due to starvation (in the paper’s experiments on BALB/c mice, “the mice had to be switched from a 4% to a 7% kcal from protein diet within the first week in order to prevent weight loss.”). Animal control officers do not allow experiments to continue if the mice are obviously starving.
[B]oth groups were implanted subcutaneously with 20,000 syngeneic murine melanoma cells (B16).
This is an unusually small number of cells. Typically, cancer researchers implant a million cells to create a syngeneic tumor. Presumably they used this small number of cells in order to ensure that some mice would not develop tumors during the 39 day experiment. As it happened, this was a lucky (canny?) choice of cell quantity: while 10 of 10 mice on the high-protein diet developed tumors during the experiment, only 9 of 10 mice on the low-protein diet did. If they had used more cells, all mice on both diets would have developed tumors; if they had used fewer cells, some mice on the high protein diet would have failed to develop tumors. Either way, the results would appear less damning for the high protein diet.
The outcomes:
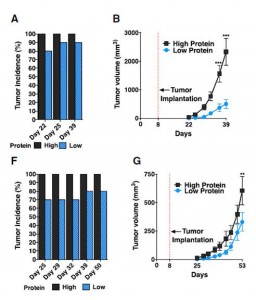
Due to the small number of cells injected, it takes at least two weeks before tumors are detectable in size (normally they would be visible in ten days). They seem to be similar in size at about two weeks after implantation.
However, when the tumors reach larger sizes, growth is impaired on the low protein diets. A mouse weighs 20 grams, and a 2000 mm3 tumor weighs 2 grams, or 10% of body weight – equivalent to a 15-pound tumor in humans. Growing a tumor of this size requires building a large amount of tissue — blood vessels, extracellular matrix, and more. The ability to construct new tissue is constrained on a protein-starved diet, so it’s not surprising that tumor growth is slower when the tumor is large and protein is severely restricted.
Animal protocols generally require that mice be sacrificed when tumors reach 2000 mm3. Extrapolating the tumor growth curves, it looks like the mice in experiment (B) would be sacrificed 5 weeks after implantation on the high protein diet, or 8 weeks after implantation on the low protein diet; in experiment (G), mice on the high protein diet would be sacrificed about 9 weeks after implantation, while mice on low protein diets would have been sacrificed about 11 weeks after implantation.
In other words, tumors still kill you, just a bit more slowly if you are starving yourself.
It’s important to note a couple of things. First, the word “starving” is appropriate. 4% to 7% protein intakes are starvation levels for mice. In a nice blog post closely relevant to this topic, Chris Masterjohn notes that a 5% protein intake completely stunts the growth of young rats:
Chris rhetorically asks: “How many of us would deliberately feed a two-year old a diet that would cause them to stop growing altogether?”
Second, as Chris also points out in the same post, such low protein intakes actually make cancer more likely in the context of exposure to mutagens. For instance, aflatoxin exposure leads to cancer (or pre-cancerous neoplasms) much more frequently in rats on low-protein diets than in rats on high-protein diets:
In this experiment, there were two diets, 5% protein and 20% protein, and two diet periods, one during exposure to aflatoxin and one afterward. Rats exposed to aflatoxin while on a 5% protein diet were far more likely to develop neoplasms than rats exposed to aflatoxin on a higher protein diet. That is, the “20-5” rats had far fewer cancers than the “5-5” rats, and the “20-20” rats had far fewer cancers than the “5-20” rats. High protein for the win!
However, once the rats had neoplasms, the tumors grew more slowly on the low-protein diet. Just as the new study found.
So, if your goal is to avoid getting cancer, it is better to eat adequate protein. If you already have cancer, or if researchers have injected you with highly metastatic melanoma cells, you can buy yourself slightly slower tumor growth by starving yourself of protein. In laboratory mice, this extends lifespan a few weeks because they are not allowed to die from cancer, but are sacrificed when tumors reach a specific size. In humans, however, cancer death commonly follows from cachexia, or wasting of lean tissue. A low protein diet might promote cachexia and accelerate cancer death in humans. It is not possible to infer from this study that there would be a clinical benefit to a low protein diet in human cancer patients.
Other Negative Effects of Low-Protein Diets
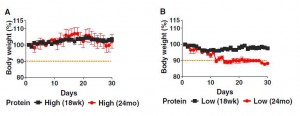
The study noted a significant negative effect of low protein diets in older mice. While young mice (18 weeks, equivalent to young adults) lost only a few percent of body weight on the starvation low protein diets, elderly mice (2 years old) wasted away on low protein diets. The data:
Both young and old mice managed to gain a bit of weight on the high protein diets, and both young and old mice lost weight on the low protein diets. The weight loss was much more severe in elderly than young mice.
Considering that wasting away commonly precedes death in the elderly, this is not a good sign for the low protein diets. The authors themselves argue that this is consistent with the NHANES finding that high protein diets become beneficial after age 65: “old but not young mice on a low protein diet lost 10% of their weight by day 15, in agreement with the effect of aging on turning the beneficial effects of protein restriction on mortality into negative effects.”
However, while I think it is clear that the dramatic weight loss in the elderly mice fed low protein is harmful, it is far from clear that the slight weight loss of the younger mice was harmless. Though they maintained their weight better than elderly mice, they may have been starving as well. To actually support the NHANES survey, the researchers should have maintained the mice on low or high protein diets for several years, and seen which group lived longer. They did not do this.
If they had, I speculate that the high protein mice would have lived longer.
Conclusion
This is a study in the line of T. Colin Campbell and other vegetarians who have tried to show that animal protein promotes cancer and mortality. These studies are unconvincing. They simply do not prove the conclusions they purport to draw.
The Perfect Health Diet takes a middle ground in regard to protein: We recommend eating about 15% protein, and argue that both high protein and low protein diets are likely to be harmful; high protein diets by accelerating aging or by making protein available to gut bacteria for fermentation, producing a less beneficial gut flora and generating nitrogenous toxins; low protein diets by starving the body of a key nutrient needed to maintain bodily functions, especially liver, kidney, and immune function.
Nothing in this study persuades me that those recommendations need revision.
References
[1] Levine ME et al. Low Protein Intake Is Associated with a Major Reduction in IGF-1, Cancer, and Overall Mortality in the 65 and Younger but Not Older Population. Cell Metabolism 19, 407–417, March 4, 2014. http://www.cell.com/cell-metabolism/retrieve/pii/S155041311400062X.













Recent Comments