Our cancer series resumes today with some tentative advice for cancer patients. (Note: This post is designed for solid tumor cancers, not blood cancers. However, most of the advice would also be applicable to blood cancers.)
This series began with Toward an Anti-Cancer Diet (Sep 15, 2011). There we advocated trying to shift cells away from the cancer phenotype via 8 anti-cancer strategies.
Future posts will explore in detail how to implement those strategies via diet and lifestyle. Today, I’m just going to give a general overview of what I would do if I had cancer.
Eat the Perfect Health Diet
This may sound self-serving, but it’s my best advice. Our diet is designed to optimize health generally, and that’s exactly what you want to do against cancer.
I said in the introduction that cancer is a disease in which cells lose their “humanness” – their proclivity to collaborate with other human cells to create a human organism. Instead, they lose recently evolved features and “remember” an identity similar to that of our distant evolutionary ancestors from the early days of multicellular life. This regression is possible because we retain the genes of our primitive evolutionary ancestors, and silencing of only a few hundred genes may cause a human cell to resemble, genetically, bacteria or fungi.
Many gut bacteria can take on two modes of behavior – a commensal or harmless phenotype, or a virulent harmful phenotype – depending on whether their environment is benign. In beneficial environments, bacteria tend to be cooperative with their host; in harsh environments, bacteria begin to look out for their own interests “selfishly,” and begin to display virulence traits which harm their host but help them move to a better environment.
Something similar may happen with “proto-cancer” cells. In a healthy environment, they are pleased to cooperate with their host – to retain their “humanness.” But in a harsh environment, they are more likely to withdraw from their neighbors and go their own way. An abused cell is more likely to become a cancer cell.
This may sound like anthropomorphization, but the metaphor is probably sound. Bruce Ames has remarked upon the fact that almost every compound is a carcinogen in large enough doses. Why? Because any unbalanced environment is harsh, and any harsh environment makes the cell more likely to develop the cancer phenotype.
It’s not only by discouraging “cancer virulence” that a good diet helps. A healthy diet also optimizes immune function.
Immune function is highly variable. Under stress, we suppress immunity so that all the body’s resources are available to meet “fight or flight” needs. Contrariwise, peaceable happiness is stimulating to immune function. A nutrient-rich diet, savory meals, happiness, calm, restful time spent in conversation – all of these things tell the body it has no pressing concerns and that available resources can be devoted to immunity and healing.
After cancer diagnosis, from a similar medical condition, those who are under stress tend to succumb to cancer, while those who are happy, cheerful, and sociable tend to recover from it. It is believed that this difference is primarily due to improved immune function in those under less stress.
I believe that a healthy, tasty diet is also a stimulant for immune function. Make your food nourishing and enjoyable.
Specific Dietary Aspects
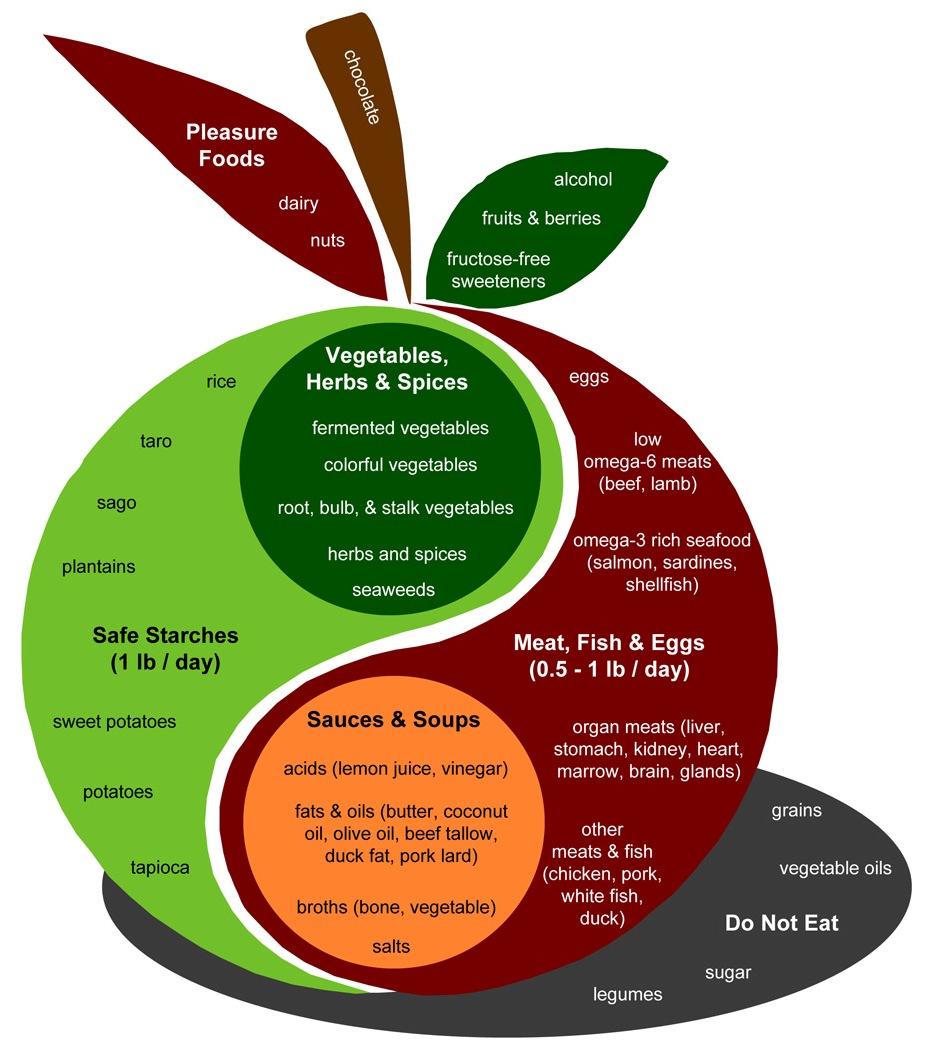
A few aspects of an anti-cancer diet deserve special mention. Let’s look at the PHD Food Plate:
Some aspects I would emphasize for cancer patients:
- Safe starches. I recommend obtaining 400 to 600 glucose calories a day, mainly from safe starches. I believe it is important to avoid a glucose deficiency, since glycosylated proteins are the means of intercellular coordination, and defects in glycosylation are characteristic of the cancer phenotype. (See, eg, this paper.) You don’t want to aggravate this with a self-induced glucose deficiency.
- Low omega-6 meats. Omega-6 fats can be very damaging to mitochondria and can promote metastasis. Our needs for them are minimal, and they are everywhere. It’s important to choose foods that minimize omega-6 levels. Among meats, prefer seafood, shellfish, and red meats; obtain eggs, milk, and organ meats from pastured and naturally raised animals. Eat tropical plant oils like coconut and palm.
- Omega-3 and omega-6 balance. The diet should include some marine sources of omega-3 fats, like salmon or sardines.
- Bone broth soups and gelatin (cooked collagen). Collagen is 30% of our body’s protein and forms much of the extracellular matrix scaffolding which is crucial to maintainance of tissue health. The extracellular matrix is broken down in cancer. An anti-cancer diet should be rich in cooked joint tissue, such as can be found in Ox Feet Broth soups. Vitamin C and sulfur, discussed below, are also required for collagen formation; be sure you’re not deficient in these.
- Fermented vegetables, yogurt, and acids. A diverse portfolio of gut bacteria can be helpful to the fight against cancer by several mechanisms. Probiotic flora from fermented foods help shield against the entry of cancer-promoting pathogens to the body through the gut; they generate by-products, like short-chain fats and vitamin K2, which have anti-cancer effects; and they can modulate immunity in a favorable direction. Acids such as vinegar and lemon juice can also favorably modify gut bacteria.
- Vegetables, herbs, and spices.Fiber is probably beneficial against cancer. Butyrate, which is produced by gut bacteria from the digestion of many types of fiber including “resistant starch” from safe starches, has anti-cancer properties. Moreover, many vegetables and traditional herbs and spices have been shown to have anti-angiogenic effects. Foods with anti-angiogenic properties include:
- Garlic.
- Tomato.
- Green tea.
- Dark chocolate / cocoa.
- Maitake mushroom.
- Bok choy.
- Kale.
- Many berries.
- Cherries.
- Ginseng.
- Turmeric.
- Oregano.
- Parsley.
- Polyphenol-rich extra virgin olive oils.
- Organ meats and egg yolks. It’s important to be well nourished, and organ meats like liver and egg yolks tend to be rich in micronutrients. They are much better than plant foods for compounds like phospholipids. In particular, choline (and its phospholipid form phosphatidylcholine) is important for methylation status and epigenetic functioning – an important element in cancer prevention.
- Sea vegetables, sea salt, and seafoods. These are good sources of trace minerals such as iodine, which is a critical anti-cancer nutrient.
In general cancer patients should focus on the foods in the apple of the PHD Food Plate more than the “pleasure foods.” However, there’s nothing wrong with some berries, dark chocolate, pistachios, and whipped cream for dessert, and some red wine with dinner. Above all, it’s important to enjoy your food. Try to obtain from every meal a sense of pleasure and well being!
Supplements
Much more could be said on this topic than I’m going to say today. One could make a very long list of supplements that might help against cancer (also a long list of those that hurt). However, the crucial five from my point of view are in our recommended supplement list:
- Vitamin D
- Vitamin K2
- Iodine
- Selenium
- Magnesium
The tricky one here is the iodine. Iodine dosage should be built up very slowly from a low level, so as not to disrupt thyroid function. (Hyperthyroidism can strongly promote cancer, and hypothyroidism can inhibit immune function and healing, so any thyroid dysfunction is a serious risk.) Start at 500 mcg or less, and increase the dose no faster than a doubling per month. If you get either hypothyroid or hyperthyroid symptoms from an increase in dose, back off a bit (eg instead of going directly from 500 mcg to 1 mg per day, go to 500 mcg and 1 mg on alternate days). Be patient, but try to build up to 12 mg/day over a 6 month period. Then stay there. Be sure to get 200 mcg/day selenium along with the iodine.
I also recommend a multivitamin, for general nourishment; and make sure there is no deficiency of vitamin C, zinc, copper, or chromium. Also, when it comes to antioxidants, more is not better. Avoid most antioxidant supplements other than glutathione, vitamin C, selenium, zinc, copper, and manganese.
For magnesium, I recommend taking a 200 mg oral supplement of magnesium citrate or a magnesium chelate. Epsom salt baths might not provide magnesium, but they can be a useful source of sulfur (in the form of sulfate) which assists collagen formation.
Vitamin C is an unusual case. It supports collagen formation, and for this purpose and to avoid a deficiency I strongly suggest taking 1 g per day. In higher doses, vitamin C may be helpful because it has anti-viral properties (see Fighting Viral Infections by Vitamin C at Bowel Tolerance, Sep 26, 2010), and most cancers are probably viral in origin. Linus Pauling, of course, advocated high doses of vitamin C – either taken orally to bowel tolerance, or intravenously. However, there are arguments on the other side. Vitamin C can protect cancer cells from immune attack, and also makes them resistant to chemotherapies. Clinical trials have not yet proven high-dose vitamin C therapy, but it may help against a subset of cancers caused by viruses sensitive to vitamin C therapy.
If sufficient amounts are not obtained from diet, then choline should be supplemented.
Intermittent Fasting, Intermittent Ketosis, Intermittent Protein Restriction
This is an extremely important cluster of strategies that are probably highly effective against cancer.
Their common trait is that all three promote autophagy, or “self-eating,” which is both a means for cells to cope with resource scarcity and a central part of the intracellular immune response.
When resources are abundant, cells allow aged organelles and junk proteins to accumulate. When resources are scarce, they turn on autophagy and digest unnecessary components, recycling the resources.
Autophagy is the dominant innate immune mechanism inside cells – the primary way cells kill bacteria and viruses.
Autophagy also recycles damaged mitochondria, which can be digested, enabling remaining healthy mitochondria to multiply. The result is a healthier mitochondrial population.
Since viruses and damaged mitochondria promote cancer, autophagy helps transform cells from the cancer phenotype back to the normal human phenotype.
Fasting, by inducing resource scarcity, promotes autophagy. Scarcity of amino acids, which can be achieved by a protein restricted diet, also promotes autophagy. And ketosis, which is part of the metabolic profile of starvation, also promotes autophagy.
Note in my section heading the shared word: “intermittent.” We don’t want to sustain fasts or protein scarcity too long; that could create malnourishment and cause more harm than good. Permanent ketosis may promote fungal infections. The most helpful course is probably to follow these strategies intermittently:
- Engage in daily intermittent fasting: eat only within a 6 to 8 hour window each day. Within the fasting period, eat some coconut oil or MCT oil to promote ketosis.
- Eat high protein for a few weeks while engaging in resistance exercise to build muscle; then low protein for a few weeks.
A Note on Ketogenic Diets
Since we wrote our book, we’ve become a bit less excited about the therapeutic potential of ketogenic diets.
Ketogenic diets have demonstrated effectiveness in brain cancers, and several considerations suggest that they would be helpful against all cancers:
- Cancer cells are dependent on glucose metabolism, a phenomenon called the Warburg effect. In ketosis, blood glucose levels can be decreased – a fall from 90 to 65 mg/dl is achievable – and reduced glucose availability should retard cancer growth.
- Mitochondria do well on ketones, and some studies had shown that provision of ketones can restore the ability of mitochondria to trigger apoptosis, or the programmed cell death of cancer cells.
It’s too early to judge, but a few scraps of data published recently have made ketogenic diets seem a bit less exciting then hoped.
First, the group of Michael Lisanti has published work suggesting that tumors can evade the metabolic restrictions of a ketogenic diet by manipulating neighboring normal cells. The idea (here is an overview) is that cancer cells release hydrogen peroxide, which causes a stress response in neighboring cells, stimulating them to release lactic acid, which the cancer cells can metabolize. This process can happen nearly as well on a ketogenic as on a normal diet, so the effectiveness of a ketogenic diet in starving the cancer cells is reduced.
The Lisanti group results are hardly conclusive – indeed so far as I know no other group has supported their claims – and there are plenty of skeptics. Jimmy Moore gathered responses from a panel of low-carb experts.
Second, clinical experience with ketogenic diets has not yet shown them to be highly effective. The sort of data we have is well represented by a recent report in Nutrition and Metabolism. Sixteen patients with advanced metastatic cancer were put on ketogenic diets. The results:
One patient did not tolerate the diet and dropped out within 3 days. Among those who tolerated the diet, two patients died early, one stopped after 2 weeks due to personal reasons, one felt unable to stick to the diet after 4 weeks, one stopped after 6 and two stopped after 7 and 8 weeks due to progress of the disease, one had to discontinue after 6 weeks to resume chemotherapy and five completed the 3 month intervention period.
The conclusion: a ketogenic diet “has no severe side effects and might improve aspects of quality of life and blood parameters in some patients.”
Clinical trials with control groups and more statistical power are needed to evaluate whether ketogenic diets have therapeutic effect. For now, I think the most prudent course is intermittent ketosis and intermittent ketogenic fasting, rather than a continuously ketogenic diet.
UPDATE: Mario makes a great point in the comments: fasting prior to chemotherapy reduces toxicity to normal cells but increases toxicity to cancer cells. It is quite likely that a ketogenic diet might have the same effect during chemotherapy. So the combination of intermittent ketogenic dieting with chemotherapy should be given consideration.
Circadian Rhythm Enhancement
Many diseases become more likely, or more severe, if circadian rhythms are disrupted. Enhancement of circadian rhythms may be therapeutic for these diseases.
I’ve blogged about circadian rhythm therapies for hypothyroidism (“Intermittent Fasting as a Therapy for Hypothyroidism,” Dec 1, 2010) and for sleep disorders, psychiatric disorders, neurodegenerative disorders, and obesity (“Seth Roberts and Circadian Therapy,” Mar 22, 2011).
Well, cancer is another disease for which circadian disruption may be damaging. The International Agency on Research on Cancer (IARC) has recently classified “shiftwork that involves circadian disruption” as “probably carcinogenic to humans.”
It’s plausible that circadian enhancement may be therapeutic for cancer. Tactics that enhance circadian rhythms include:
- Exposure to mid-day sunlight.
- Sleeping in total darkness during hours of darkness.
- Confining eating to daylight hours.
- Socializing – especially, looking at faces and talking – during daylight hours. Seth Roberts found that looking at images of human faces can substitute for actual socializing.
- Exercising during daylight hours. Even low-level activity – like standing instead of sitting – helps.
- In people who are melatonin deficient due to a brain immune response, supplementation of melatonin just before bedtime.
Curiously, circadian rhythm disruption seems to make chemotherapy more effective. Also, timing treatments to match circadian rhythms may double their effectiveness.
Exercise and Other Lifestyle Factors
A number of lifestyle factors are important for cancer recovery. David Servan-Schreiber’s Anti-Cancer has an excellent overview of the evidence.
A recent study in the Lancet found that every additional 15 min of daily exercise beyond 15 min a day reduced all-cancer mortality by 1%. Exercise appears to be therapeutic even for late stage cancers. A meta-review found that two and a half hours of exercise a week could lower a breast cancer patient’s risk of dying or cancer recurrence by 40 percent, and could reduce a prostate cancer patient’s risk of dying from the disease by about 30 percent.
However, exercise should not be exhausting. Rather, it should be restful and relaxing; or build muscle. Resistance exercise on the “Body by Science” model of one intense workout per week, with more time spent in restful recovery than in stress, is probably a good strategy. Long walks outdoors in nature, and relaxing exercises like yoga or tai chi, are also great approaches to cancer therapy.
Being sociable, happy, calm, and optimistic are all important factors for cancer recovery. Those who have companions they love, and a purpose for living that makes them happy, have the best prognosis. Be grateful for what you have, and make your body understand that life is worth living.
Dealing with Anorexia and Nausea
Anorexia and nausea can seriously impair the ability of cancer patients to eat a nourishing diet and maintain their strength.
I haven’t had time to research this aspect of the disease yet, but there do seem to be some dietary and lifestyle interventions that help.
For instance, exercise can correct anorexia.
Among dietary interventions, ginger has been reported to reduce chemotherapy-induced nausea, reducing incidence in one study from 93% to 55%. (Hat tip: Healthy Fellow.)
Ginger teas are a traditional Asian folk remedy. Slice some ginger root in water, boil it on the stove, add some rice syrup for sweetness, and drink up!
Under-Utilized Therapies
There are a few therapies which are rarely prescribed, but might be more helpful than chemotherapies in treating cancer:
- Low-dose naltrexone.
- Anti-viral drugs.
- Anti-fungal therapies.
Low-dose naltrexone is taken at night before bed. It temporarily blocks opioid receptors, which leads the body to increase production of endorphins and enkephalins – immune compounds which interact with opioid receptors. The following day, the naltrexone is gone and the opioid receptors are working again, but the endorphins are still around. Taking LDN thus increases endorphin levels. Endorphins inhibit cancer proliferation, and may enhance anti-cancer immunity. Here is a recent paper on anti-proliferative effects of LDN against ovarian cancer: http://pmid.us/21685240. Here is a recent paper on LDN plus alpha lipoic acid as a therapy against pancreatic cancer: http://pmid.us/20042414. For a general overview, see http://lowdosenaltrexone.org/.
Viruses cause or contribute to most cancers, and thus anti-viral drugs have great potential. A few cancer-causing viruses are famous, such as the Human Papilloma Virus for which there is a vaccine; however, most of the viruses that cause cancer remain unknown, though we know they exist because genetic mutations that impair viral immunity greatly increase cancer incidence.
Mario Renato Iwakura recently sent me a link to a paper that nicely illustrates the potential of antiviral therapies against cancer. Cytomegalovirus, also known as human herpes virus 5, is a common virus that infects 40% of adults worldwide and 50% to 80% of Americans. However, it is found in almost 100% of human tumors. It seems to be difficult to get cancer if you haven’t been infected by cytomegalovirus.
From the paper abstract:
Medulloblastomas are the most common malignant brain tumors in children…. Human cytomegalovirus (HCMV) is prevalent in the human population and encodes proteins that provide immune evasion strategies and promote oncogenic transformation and oncomodulation…. Remarkably, all of the human medulloblastoma cell lines that we analyzed contained HCMV DNA and RNA and expressed HCMV proteins at various levels in vitro. When engrafted into immunocompromised mice, human medulloblastoma cells induced expression of HCMV proteins. HCMV and COX-2 expression correlated in primary tumors, cell lines, and medulloblastoma xenografts. The antiviral drug valganciclovir and the specific COX-2 inhibitor celecoxib prevented HCMV replication in vitro and inhibited PGE2 production and reduced medulloblastoma tumor cell growth both in vitro and in vivo.
Tumor growth declined by 72% when treated with Valcyte (valganciclovir) and an NSAID drug. A press release notes that these drugs have “relatively good adverse effect profiles” and that “antiviral drugs are selective and largely affect infected cells.”
Yet another antimicrobial approach that may be helpful against cancer is antifungal therapy. Most cancer patients develop systemic fungal infections, and fungal infections such as Candida promote metastasis and tumor growth, and may also suppress anti-cancer immunity. An effective antifungal therapy may significantly retard cancer progression.
Conclusion
Much more remains to be said, and it’s certain that we’ll refine these suggestions after more thoroughly studying the literature. But I think this basic approach to an anti-cancer diet can’t be too far wrong.
Our prayers and best wishes go out to all those who are battling cancer.











Recent Comments