In the earlier two posts of this series (HDL and Immunity, April 12; HDL: Higher is Good, But is Highest Best?, April 14), we established that HDL is central to the immunity and toxin clearance, and that it’s probably desirable to have more of it than our body’s natural levels, since we are in a more pathogen-and-toxin-rich environment than the Paleolithic and evolution hasn’t caught up to the situation.
The question is: how?
Disease Can Upregulate HDL
Chris Kresser left two great comments (here and here):
I tend to view HDL >85 or 90 in the presence of other inflammatory or immunological markers as a potential sign of infection or immune dysregulation.
I don’t have the reference handy, but I came across a study associating elevated HDL and CRP (occurring together) with INCREASED risk of heart disease….
I frequently see HDL >100 in patients with several other markers of inflammation, such as elevated CRP, ferritin, WBC, monocytes, etc.
Yes, indeed; as one review of HDL and heart attacks states, “many patients who experience a clinical event have normal, or even high, levels of HDL cholesterol.” [1]
Heart attacks result from a high burden of infected atherosclerotic lesions. When the body is fighting infections, it upregulates its defense mechanisms, including HDL.
Mario added a great comment along this line:
This could explain why runners have higher levels of HDL: to fight infections that abound among them!
And, the fact that pathogen-fighting HDL particles do not go back to the liver can explain why the half-life of HDL in runners is much higher than in sedentaries http://www.ncbi.nlm.nih.gov/pubmed/6748208.
These facts lead us toward one possible strategy for raising HDL: swallow a lot of pathogens!
Our Strategy: Benign Hormetic Stress
But this isn’t likely be desirable. Higher HDL may do some good, but the pathogens are likely to do a lot more harm.
So we have to look at tactics for raising HDL that do more good than harm. I think it’s useful to classify tactics in three groups:
- Beneficial Methods. These methods have no known toxicity, but cause the body to increase HDL levels – perhaps because of an association with danger in our evolutionary past.
- Mildly Toxic, Plausibly Beneficial Methods. These methods have some toxicity, but there is a plausible case to be made that the toxicity is innocuous or insignificant, so that the benefits of higher HDL will outweigh the harms.
- Damaging Methods. Intentionally swallowing HDL-increasing pathogens or toxins is probably a bad strategy most of the time, and should be avoided.
I’ll look at tactics one by one.
Coconut Oil-Induced Ketosis
One of the most powerful, and probably also benign, ways to raise HDL is intermittent fasting or ketogenic dieting, with ketosis enhanced by the use of coconut oil or MCT (medium-chain triglyceride) oil.
Ketosis stimulates the ketone receptor GPR109A, which strongly induces HDL synthesis. GPR109A is better known as the receptor on which niacin acts to raise HDL, but its physiological ligand was not known until recently when it was found to be the ketone beta-hydroxy-butyrate. [2] It looks like any time a human goes into ketosis, HDL is upregulated.
Why we evolved a mechanism to increase HDL during ketosis is not known. However, it’s easy to imagine plausible stories. Ketosis would have been a frequent event in the Paleolithic, since most hunter-gatherers probably ate low-carb diets. However, ketosis would have been associated with times of stress:
- Ketosis occurs during fasting, and involuntary fasting is a threat to health that forces eating of marginal foods from which infection risks are high.
- Ketosis can also be induced by a lack of carb-containing plant foods; this would naturally lead to a shortage of animal foods, and famine, soon after. Famine depresses immunity and increases risk of infection.
- Drought was probably a common cause of both lack of carb-rich foods and famine. Drought would tend to force reliance on marginal, polluted or infected water sources.
In the modern world, we control our food intake and can generate ketosis safely without ever reaching a famine state that significantly depresses immunity. There are safe ways to activate GPR109A via intermittent ketogenic dieting with minimal risk of ill effects.
I believe the chief risks from ketogenic dieting are:
1. Promotion of protozoal and fungal infections. While ketogenic dieting is helpful against bacterial and viral infections, fungi and protozoa are eukaryotes who can metabolize ketones in their mitochondria. In fact, because ketones are water-soluble small molecules and diffuse into pathogen mitochondria, while glucose and fatty acids are chaperoned through the human body by transport molecules, ketones are a uniquely available energy substrate for parasitic fungi and protozoa. Moreover, glucose is a major resource for the immune defense against these pathogens, and induction of ketosis by carb restriction can diminish immunity against protozoa and fungi. Since protozoal infections such as Toxoplasma gondii and fungal infections such as Candida are now common, each afflicting perhaps 30% of the population or more, this is a major concern.
2. Ketosis induced through severe carb and protein restriction may trigger the dangers of zero-carb dieting. I’ve done a series on this (it started here).
The solution is to achieve ketosis intermittently, through tactics like daily intermittent fasting, and to induce it positively through the consumption of short-chain fats such as are found in coconut oil or MCTs, or through leucine supplementation. A healthy diet, ketogenic or not, should meet our recommendations of at least 200 carb calories from starches and at least 600 calories from carbs plus protein.
Physical Activity
Exercise is another method for raising HDL that seems entirely healthful.
In the evolutionary environment, continuous exertion probably signaled danger: either a difficult hunt or, more likely, some form of warfare with other humans. In either case, injury and a need for wound repair was a likely prospect. Breaching of the skin barrier by wounds mean infections. Since HDL plays a role in wound repair and infection resistance, it would make sense to upregulate HDL production during exertion.
In one 12-week trial, HDL was raised by 24.8% on a moderate-intensity walking program and by 20.9% on a high-intensity walking program. [3]
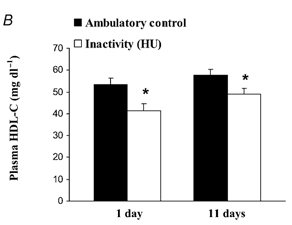
In the evolutionary milieu, sitting for 16 hours a day would have indicated a lack of danger and little need for HDL. It turns out that daily sitting time strong predicts low HDL – and it only takes a day for HDL levels to adjust. In rats, 16 hours of daily inactivity caused a 20-25% drop in HDL levels by the end of the first day [4]:
The same phenomenon occurs in humans: 20 days of bed rest leads to a 20% reduction in HDL [5].
Resistance training also helps, but perhaps not as much as reduced sitting time. Obese sedentary women raised HDL by 15% following a 9-week, 3 times per week resistance training protocol. [6]
Overall, the most effective way to raise is HDL through activity is simply to reduce the daily time spent sitting and increase the time spent standing or walking.
Sitting is also a major risk factor for obesity, diabetes, and cardiovascular disease. [7] So it looks like high activity levels are strongly health-improving.
Until recently I was sitting or sleeping about 23 hours a day, which can’t be healthful. To repair that I recently built a standing desk. Now I stand, kneel (on a padded bench), or half-kneel half-stand throughout my working hours. I strongly recommend a standing desk as an effective way to increase HDL.
High-Fat Diets and Dairy Fat Consumption
When long-chain fats are eaten, they are transported from the intestine by particles called chylomicrons. Researchers injected radiolabeled chylomicrons into rats to determine the fate of the components. They found that chylomicrons frequently turn into HDL:
Catabolism of chylomicrons is associated with a rapid transfer of phospholipid, apoA-I, and possibly apoA-IV into HDL. Chylomicron phospholipid appears to give rise to vesicles which are probably incorporated into preexisting HDL. Chylomicron surface components may be an important source of plasma HDL. [8] (Hat tip CarbSane.)
Which long-chain fats are best? A case can be made for dairy fats.
There is a clear association between eating dairy fats and having high HDL. Blood levels of trans-palmitoleic acid, an omega-7 trans-fat obtainable only by eating milk products from ruminants, is strongly associated in prospective cohort studies with higher HDL. [9b] Feeding experiments in guinea pigs confirm that butter oil increases HDL. [9]
Seth Roberts cut his coronary artery calcification score by 24% by eating a half-stick of butter per day; perhaps butter’s HDL-raising property deserves the credit. High dairy fat consumption is associated with improved health in prospective cohort studies, for instance much lower rates of diabetes and lower CRP levels. [9b]
The reason dairy fats work is uncertain. The mechanism could be via chylomicron breakdown, and other fats might work nearly as well. People who eat the most dairy fats probably eat high-fat diets that are low in omega-6 fats, and omega-6 fats reduce HDL; so the dairy fats could just be a marker for high-fat low-omega-6 diets. However, it’s possible that the ruminant trans-fat CLA is especially beneficial. It might not hurt to copy Seth, and eat a lot of butter.
Alcohol
Interestingly, drinking alcohol may be a healthful way to raise HDL. We’ve previously discussed epidemiological evidence for health benefits from drinking and the matter of how to drink safely (Is It Smart to Drink?, Sep 9, 2010), but didn’t discuss alcohol’s effect on HDL.
Well, it’s significant. Alcohol increases HDL-C level, with higher doses of ethanol leading to higher HDL levels and lower rates of coronary artery disease. This works as long as there is no liver damage. Once liver damage begins, alcohol lowers HDL. [10]
This is good news because alcohol alone does not damage the liver – only the combination of alcohol with polyunsaturated fats – either omega-6 or omega-3 will do. As long as alcohol is consumed only with saturated fats, it is likely to be beneficial to health.
Here’s some numbers relating alcohol dose to HDL increase:
- One beer per day raised HDL by 4.4% without affecting other lipid parameters. [11]
- A half-bottle of wine per day (containing 39 g ethanol) raised HDL by 17% without affecting other lipid parameters. [12]
It is possible that red wine is particularly beneficial for HDL due to certain plant compounds that accompany the alcohol. [13]
In an analysis of the MRFIT trial, alcohol’s HDL-raising effect was found to be responsible for half of its benefits for mortality from coronary heart disease. [14] It appears that alcohol’s other health benefits, from its glucose-lowering effect to its stress-relieving effect, are less important for health than its HDL-raising effect.
Niacin
Niacin supplementation is the most common doctor-prescribed way to raise HDL. Dr. William Davis of Track Your Plaque fame is an ardent advocate of niacin, as are many other cardiologists.
Niacin increases HDL the same way ketones do, by activating the ketone receptor GPR109A. Gram doses of niacin are roughly equally effective with tablespoon doses of coconut oil in raising HDL. For instance, four grams of niacin per day for 6 weeks raised HDL levels by 50%. [15]
However, there are two key differences:
1. Niacin is toxic whereas ketones are not.
2. Ketones diffuse throughout the body whereas niacin binds certain cells, notably fat cells, preferentially and this concentrates its toxicity.
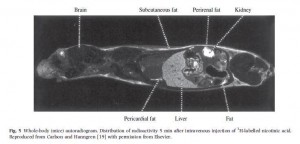
The localization of niacin to fatty tissues is clear in this radiogram taken 5 minutes after injection of radioactive-labeled niacin to a mouse [16]:
Major sites of niacin binding are the skin, the liver, and the fat surrounding the kidney. This is why these are sites of niacin toxicity: the toxins from niacin conversion are localized here.
Skin flushing is the most obvious sign of niacin toxicity, but organs can also be damaged. As one review states, “Unfortunately, when used as a pharmaceutical, niacin has more than its share of drug toxicities, including hepatotoxicity, gastric toxicity, glucotoxicity, and, most commonly, skin toxicity.” [17]
Niacin toxicity results from the manner in which it is converted to the active forms of vitamin B3, NAD and NADP. Niacinamide, an alternative form of vitamin B3, is converted to NAD and NADP without toxicity, but does not stimulate the GPR109A receptor and does not raise HDL levels.
Time-release niacin is especially prone to poisoning the liver. [18] The liver’s main niacin disposal pathway can only metabolize a small amount of niacin at a time. Time-release niacin causes more niacin to pass through this high-toxicity liver pathway.
Another issue with niacin is that NAD is the rate-limiting vitamin for bacterial metabolism. Excess vitamin B3 intake, therefore, promotes bacterial infections.
In general, I consider niacin to be an alternative to coconut oil-driven ketosis rather than a complement to it. Both niacin and ketones act on the same receptor, and the HDL increases from coconut oil alone are so large (commonly to 120 mg/dl or higher) that adding niacin on top would be gilding the lily.
Since benefits from niacin against atherosclerosis probably come either from HDL increases or from other effects of activating GPR109A [19], it is likely that coconut oil delivers all or nearly all the benefits of niacin.
In most cases, due to its lack of toxicity, coconut oil should be preferred. The exception would be people who have significant protozoal or fungal infections but not bacterial infections. Since ketones feed the former while niacin feeds the latter, such people may benefit from niacin instead of coconut oil.
Some other HDL-raising factors
In general, good nutritional status supports high HDL levels. In some populations, multivitamin and multimineral supplements have been shown to raise HDL. [20]
Micronutrients that are beneficial may include vitamin C, taurine, and glycine. Bile acids are made from cholesterol using vitamin C and are then conjugated with taurine and glycine. Bile duct blockage tends to lower HDL and providing bile-supporting nutrients like vitamin C [21] and taurine [22, 23] can under some circumstances raise HDL. Glycine is richly present in gelatin (cooked collagen), and taurine in uncooked or rare meats.
Plant fiber and polyphenols have been reported to raise HDL. For instance, a polyphenol-rich carob fiber was found to raise HDL by 7% while lowering LDL [24], and psyllium has been reported to raise HDL [25]. It is unclear to me whether this is a beneficial pathway or not. On the one hand, butyrate and other volatile fatty acids from gut flora may stimulate the ketone receptor. On the other hand, many of these polyphenols are directly toxic, and fiber increases gut bacterial populations and endotoxin flux into the body. HDL may be upregulated because it has more toxins to clear.
Higher potassium excretion is associated with higher HDL, suggesting that high intake of potassium-rich foods like potatoes, bananas, and vegetables might raise HDL. [26] Since potassium-rich foods are also usually fiber-rich, this association may be mediated by short-chain fats from fermentation of fiber by gut bacteria. But potassium is a nutrient low-carb dieters can easily become deficient in, so it may be worth tending to.
Some Things to Avoid
Dietary components that promote lipid peroxidation, including fructose, omega-6 fats, and trans-fats, lower HDL levels. Smoking also lowers HDL. [27]
A Japanese study found that “Concerning dietary habits, total cholesterol was lower by a mean of 13 mg/dl (0.34 mmol/L), triglycerides lower by 40 mg/dl (0.45 mmol/L), and HDL-cholesterol higher by 5 mg/dl (0.13 mmol/L) in the group who ate 7 or more Japanese-style meals in the 9 meals during 3 days than in the group who ate 3 or less Japanese-style meals in the 9 meals.” [27]
Japanese-style meals are low-toxicity and essentially Perfect Health Diet compliant. The alternative is probably western style food high in wheat, vegetable oils, and sugar.
Conclusion
HDL can be raised in destructive ways – such as ingestion of toxins or pathogens – but there are healthy ways to raise HDL.
I believe the following four ways are healthiest, and are sufficient to optimize HDL levels:
- Eat a nourishing diet rich in saturated and monounsaturated fat, especially dairy fat, but low in omega-6 fats, fructose, and other toxins. In short: eat the Perfect Health Diet.
- Be physically active. Be on your feet as much as possible; favor a standing desk over sitting. Do resistance exercise or other intense exercise occasionally.
- Engage in intermittent fasting, and consume a lot of coconut oil, coconut milk, or MCTs to stimulate the ketone receptor.
- Drink alcoholic beverages – but only when consuming meals low in polyunsaturated fats. Drink up when you eat beef, but be cautious when the entrée is salmon.
Niacin, the most effective pharmaceutical for raising HDL, has some toxicity and is probably inferior to coconut oil and intermittent fasting except in people with protozoal or fungal infections.
Our best wishes for high HDL!
Related posts:
- HDL and Immunity, April 12, 2011
- HDL: Higher is Good, But is Highest Best?, April 14, 2011
References
[1] Navab M et al. HDL and cardiovascular disease: atherogenic and atheroprotective mechanisms. Nat Rev Cardiol. 2011 Apr;8(4):222-32. http://pmid.us/21304474.
[2] Ahmed K et al. GPR109A, GPR109B and GPR81, a family of hydroxy-carboxylic acid receptors. Trends Pharmacol Sci. 2009 Nov;30(11):557-62. http://pmid.us/19837462.
[3] Spate-Douglas T, Keyser RE. Exercise intensity: its effect on the high-density lipoprotein profile. Arch Phys Med Rehabil. 1999 Jun;80(6):691-5. http://pmid.us/10378497.
[4] Bey L, Hamilton MT. Suppression of skeletal muscle lipoprotein lipase activity during physical inactivity: a molecular reason to maintain daily low-intensity activity. J Physiol. 2003 Sep 1;551(Pt 2):673-82. http://pmid.us/12815182.
[5] Yanagibori R et al. The effects of 20 days bed rest on serum lipids and lipoprotein concentrations in healthy young subjects. J Gravit Physiol. 1997 Jan;4(1):S82-90. http://pmid.us/11541183.
[6] Costa RR et al. Effects of resistance training on the lipid profile in obese women. J Sports Med Phys Fitness. 2011 Mar;51(1):169-77. http://pmid.us/21297577.
[7] Hamilton MT et al. Role of low energy expenditure and sitting in obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease. Diabetes. 2007 Nov;56(11):2655-67. http://pmid.us/17827399.
[8] Tall AR et al. Metabolic fate of chylomicron phospholipids and apoproteins in the rat. J Clin Invest. 1979 Oct;64(4):977-89. http://pmid.us/225354.
[9] Rice BH et al. Ruminant-produced trans-fatty acids raise plasma total and small HDL particle concentrations in male Hartley guinea pigs. J Nutr. 2010 Dec;140(12):2173-9. http://pmid.us/20980644.
[9b] Mozaffarian D et al. Trans-palmitoleic acid, metabolic risk factors, and new-onset diabetes in U.S. adults: a cohort study. Ann Intern Med. 2010 Dec 21;153(12):790-9. http://pmid.us/21173413.
[10] Lakshman R et al. Is alcohol beneficial or harmful for cardioprotection? Genes Nutr. [Epub ahead of print] http://pmid.us/20012900.
[11] Thornton J et al. Moderate alcohol intake reduces bile cholesterol saturation and raises HDL cholesterol. Lancet. 1983 Oct 8;2(8354):819-22. http://pmid.us/6137650.
[12] McConnell MV et al. Effects of a single, daily alcoholic beverage on lipid and hemostatic markers of cardiovascular risk. Am J Cardiol. 1997 Nov 1;80(9):1226-8. http://pmid.us/9359559.
[13] Brien SE et al. Effect of alcohol consumption on biological markers associated with risk of coronary heart disease: systematic review and meta-analysis of interventional studies. BMJ. 2011 Feb 22;342:d636. http://pmid.us/21343206.
[14] Suh I et al. Alcohol use and mortality from coronary heart disease: the role of high-density lipoprotein cholesterol. The Multiple Risk Factor Intervention Trial Research Group. Ann Intern Med. 1992 Jun 1;116(11):881-7. http://pmid.us/1580443.
[15] Carlson LA, Hamsten A, Asplund A. Pronounced lowering of serum levels of lipoprotein Lp(a) in hyperlipidaemic subjects treated with nicotinic acid. J Intern Med 1989; 226: 271–6.
[16] Carlson LA. Nicotinic acid: the broad-spectrum lipid drug. A 50th anniversary review. J Intern Med. 2005 Aug;258(2):94-114. http://pmid.us/16018787.
[17] Dunbar RL, Gelfand JM. Seeing red: flushing out instigators of niacin-associated skin toxicity. J Clin Invest. 2010 Aug 2;120(8):2651-5. http://pmid.us/20664168.
[18] Bassan M. A case for immediate-release niacin. Heart Lung. 2011 Mar 15. [Epub ahead of print] http://pmid.us/21414665.
[19] Lukasova M et al. Nicotinic acid inhibits progression of atherosclerosis in mice through its receptor GPR109A expressed by immune cells. J Clin Invest. 2011 Mar 1;121(3):1163-73. http://pmid.us/21317532.
[20] Li Y et al. Effects of multivitamin and mineral supplementation on adiposity, energy expenditure and lipid profiles in obese Chinese women. Int J Obes (Lond). 2010 Jun;34(6):1070-7. http://pmid.us/20142823.
[21] Yanai H, Morimoto M. Effect of ascorbate on serum lipids and urate metabolism during exhaustive training. Clin Sci (Lond). 2004 Jan;106(1):107-9. http://pmid.us/12927020.
[22] Choi MJ. Effects of dietary taurine supplementation on plasma and liver lipids in OVX rats fed calcium-deficient diet. Nutr Res Pract. 2008 Spring;2(1):13-6. http://pmid.us/20126359.
[23] Elvevoll EO et al. Seafood diets: hypolipidemic and antiatherogenic effects of taurine and n-3 fatty acids. Atherosclerosis. 2008 Oct;200(2):396-402. http://pmid.us/18242615.
[24] Ruiz-Roso B et al. Insoluble carob fiber rich in polyphenols lowers total and LDL cholesterol in hypercholesterolemic sujects. Plant Foods Hum Nutr. 2010 Mar;65(1):50-6. http://pmid.us/20094802.
[25] Giacosa A, Rondanelli M. The right fiber for the right disease: an update on the psyllium seed husk and the metabolic syndrome. J Clin Gastroenterol. 2010 Sep;44 Suppl 1:S58-60. http://pmid.us/20616745.
[26] Ishikawa M et al. Taurine’s health influence on Japanese high school girls. J Biomed Sci. 2010 Aug 24;17 Suppl 1:S47. http://pmid.us/20804624.
[27] Hata Y, Nakajima K. Life-style and serum lipids and lipoproteins. J Atheroscler Thromb. 2000;7(4):177-97. http://pmid.us/11521681.











Recent Comments