On Wednesday a new paper reported that higher levels of long-chain omega-3 fats (EPA, DPA, and DHA) in blood are associated with a 43% increased risk of prostate cancer and a 71% increased risk of aggressive prostate cancer. [1] This built on earlier work by the same group. [2] In a press release, the authors stated:
“We’ve shown once again that use of nutritional supplements may be harmful,” said Alan Kristal, Dr.P.H., the paper’s senior author …
“[W]e have confirmed that marine omega-3 fatty acids play a role in prostate cancer occurrence,” said corresponding author Theodore Brasky, Ph.D.
They sound confident! Is there anything to it, and should it affect our dietary advice?
Mechanisms Linking Omega-3 Fats to Cancer
In our book and on this blog, we’ve already discussed two mechanisms linking excessive omega-3 intake to cancer risk.
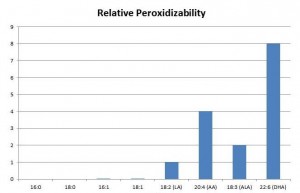
First, there is the issue of lipid peroxidation. Of all fatty acids, long-chain omega-3 fats are the most readily peroxidized:
Peroxidation of PUFA generates highly toxic compounds, such as aldehydes, which mutate DNA and turn proteins into advanced lipoxidation end products (ALEs). [3, 4] These lipid peroxidation products have been implicated as causal factors in cancer. [5]
Second, oxidation products of DHA promote angiogenesis – the creation of new blood vessels to feed tumors. These products make cancers grow rapidly. I’ve blogged about this (DHA and Angiogenesis: The Bottom Line, May 4, 2011; Omega-3s, Angiogenesis and Cancer: Part II, April 29, 2011; Omega-3 Fats, Angiogenesis, and Cancer: Part I, April 26, 2011).
So there are known mechanisms by which the long omega-3s in fish oil may promote cancer.
The Brasky et al Papers
The new study by Brasky et al measured omega-3 fat levels in plasma phospholipids. Thus, it doesn’t measure any omega-3s in cells, only omega-3s in serum particles like LDL, HDL, and VLDL; and even in those particles it excludes omega-3 fats found in triglycerides.
This is a very different biomarker than the Omega-3 Index of William Harris, which looks at the omega-3 phospholipids in red blood cell membranes. [6] This biomarker might behave quite differently than the Omega-3 Index.
The study measured plasma phospholipid omega-3s in a group of people, then followed them for 6 years or so to see who developed cancer. Here are the group averages [1]:
Statistically the most reliable data is in the no cancer vs total cancer comparison. There we find that subjects who went on to develop prostate cancer averaged 3% more DHA and 4% more EPA+DPA+DHA in plasma phospholipids than those who didn’t develop cancer.
Does This Variation Reflect Dietary Intake?
Chris Kresser kindly sent a link to an analysis of the study published at LecturePad by William Harris: “Omega-3 Fatty Acids and Risk for Prostate Cancer.” Harris tells us how to translate the plasma numbers to the corresponding Omega-3 Index numbers:
Based on experiments in our lab, the lowest quartile would correspond to an HS-Omega-3 Index of <3.16% and the highest to an Index of >4.77%).
Even the top quartile of the Brasky et al subjects had quite low omega-3 levels:
In Framingham, the mean Omega-3 Index of participants who were not taking fish oil supplements was 5.2% and for those taking supplements, it was 7.5% [7]. Both of these numbers are considerably higher than the values reported by Braskey et al., even in their highest quartile.
The trial asked its participants not to take supplements, and it looks like they drew a study population whose fish intake was much lower than that of Framingham, Massachusetts, residents.
If dietary omega-3 intake was low in all subjects and varied only slightly among participants, how do we know that this biomarker is related in any way to dietary intake? There could be other factors – genetics, oxidative environment, omega-6 fat intake, antioxidant intake, changes in the proportions of VLDL, LDL, and HDL, to name a few – that affect this biomarker.
Does High Dietary Intake Lead to More Cancer?
If high dietary intake of omega-3s caused more cancer, we would expect cultures that consume lots of fish oil to have higher prostate cancer rates. But epidemiological studies have found that high omega-3 intakes seem to be associated with low cancer rates. For instance, the Japanese eat eight times more omega-3 fatty acids than Americans and their blood levels are twice as high, yet the prostate cancer rates are only one-sixth the American rate.
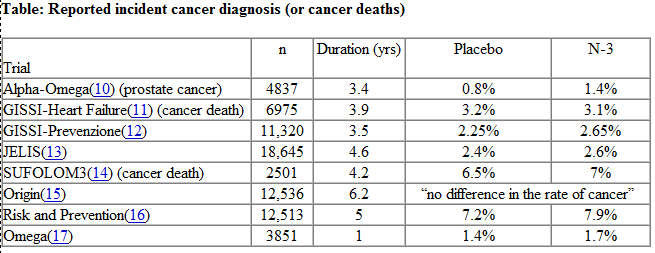
Of course, there are many confounders in epidemiological studies. Harris helpfully provides a summary of clinical trials in which fish oil was provided as part of the study and cancer outcomes measured:
Although none of these studies produced a statistically significant link between omega-3 intake and cancer, incidence of cancer diagnosis or death was increased in every one of the clinical trials except the GISSI-Heart Failure study and perhaps the Origin study. A meta-analysis might find a small cancer promoting effect of omega-3s.
UPDATE: Vladimir Heiskanen points me to an interesting paper in which the effect of dietary fatty acids on cancer metastasis was examined. Colon carcinoma cells were injected into the portal vein (which leads from intestine to liver) of rats and 3 weeks later rats were sacrificed and their livers were examined for metastases. The rats were on three diets — low-fat, high omega-6 (safflower oil), high omega-3 (fish oil). The results:
At 3 weeks after tumor transplantation, the fish oil diet and the safflower oil diet had induced, respectively, 10- and 4-fold more metastases (number) and over 1000- and 500-fold more metastases (size) than were found in the livers of rats on the low-fat diet. [7]
I wish they’d used a saturated fat or monounsaturated fat diet, rather than a low-fat diet, as the control, as this would have clarified that polyunsaturates specifically promote metastasis; in the study the rats’ food was mixed with fish oil or safflower oil, greatly increasing the fat fraction and decreasing the carbohydrate, protein, and micronutrient fractions, so the control diet deviates in many respects from the high-PUFA diets. However, the results are consistent with the idea that fish oil is more cancer-promoting than the less peroxidizable safflower oil, perhaps because of the unique pro-angiogenic effects of DHA products.
Conclusion
There might be biological contexts in which omega-3 fats promote cancer.
This doesn’t mean we should refrain from eating omega-3 fats. Cardiovascular disease causes more deaths than cancer, and omega-3 fats are protective against CVD.
However, I think these studies support the PHD advice:
- Eat enough oily marine fish to achieve omega-6 and omega-3 balance;
- Minimize omega-6 intake so that omega-6 and omega-3 balance is achieved at the lowest possible intake of polyunsaturated fats.
All nutrients can be eaten in excess, and omega-3 fats surely fall into this category. The right amount of oily fish is probably about one to two meals per week.
References
[1] Brasky TM et al. Plasma Phospholipid Fatty Acids and Prostate Cancer Risk in the SELECT Trial. J Natl Cancer Inst. 2013 Jul 10. [Epub ahead of print] http://pmid.us/23843441.
[2] Brasky TM et al. Serum phospholipid fatty acids and prostate cancer risk: results from the prostate cancer prevention trial. Am J Epidemiol. 2011 Jun 15;173(12):1429-39. http://pmid.us/21518693.
[3] Hulbert AJ et al. Life and death: metabolic rate, membrane composition, and life span of animals. Physiological Reviews 2007 Oct;87(4):1175–213, http://pmid.us/17928583.
[4] Hulbert AJ. Metabolism and longevity: is there a role for membrane fatty acids? Integretive and Comparative Biology 2010 Nov;50(5):808–17, http://pmid.us/21558243.
[5] Nair U, Bartsch H, Nair J. Lipid peroxidation-induced DNA damage in cancer-prone inflammatory diseases: a review of published adduct types and levels in humans. Free Radic Biol Med. 2007 Oct 15;43(8):1109-20. http://pmid.us/17854706.
[6] Harris WS, Von Schacky C. The Omega-3 Index: a new risk factor for death from coronary heart disease? Prev Med. 2004 Jul;39(1):212-20. http://pmid.us/15208005.
[7] Griffini P et al. Dietary omega-3 polyunsaturated fatty acids promote colon carcinoma metastasis in rat liver. Cancer Res. 1998 Aug 1;58(15):3312-9. http://pmid.us/9699661.












Recent Comments