I also do work in economics and one of my favorite economics blogs is Evolving Economics by Jason Collins. He has an interest in biology and Paleo diets and recently linked to an interesting train of thought from evolutionary biologist Michael Rose.
Here is a summary from Peter Turchin, who adopted a Paleo diet this spring after talking to Rose:
We think of people having ‘traits,’ but actually we change quite dramatically as we age. … As an extreme example, consider reproductive ability, something of great interest to evolution. Humans do not reproduce until they reach a fairly advanced age of maturation (puberty). Young adults are not very good mothers or fathers, but they improve with age during their twenties. After that reproductive ability declines and eventually disappears. …
Ability to digest certain foods can also be age-dependent. I have already mentioned the ability to digest lactose, the sugar present in milk. Before we domesticated animals such as cows and sheep, only very young humans had this ability. Natural selection turned this ability off in adults because they never needed it (and it would be wasteful to continue producing the enzyme lactase that aids in the digestion of milk sugar). …
Because abilities to do something at the age of 10, 30, 50, etc. are separate (even if correlated) traits, they evolve relatively independently of each other. When grains became a large part of the diet, the ability of children to digest them (and detoxify the chemical compounds plants put into seeds to protect them against predators such as us) became critical. If you don’t have genes to help you deal with this new diet, you don’t survive to adulthood and don’t leave descendants. In other words, evolution worked very hard to adapt the young to the new diet. On the other hand, the intensity of selection on the old (e.g., 55 years old) was much less – in large part, because most people did not live to the age of 55 until very recently. …
The striking conclusion from this argument is that older people, even those coming from populations that have practiced agriculture for millennia, may suffer adverse health effects from the agricultural diet, despite having no problems when they were younger.
This is an intriguing argument. Several aspects of it are well supported: there has been recent evolution to enable people to cope with toxic diets, and there are substantial changes in how we respond to food as we age.
Recent Genetic Evolution
We know that there has been recent evolution for greater tolerance to evolutionarily novel foods such as wheat. This is (presumably) why peoples with a long history of grain agriculture are less obese and diabetic on “western” diets than people with a long history of eating healthy foods.
The Pacific islanders are a great example. The world’s highest obesity rates are in the Pacific – for instance, in the Kosrae district of Micronesia, 88% of adults are overweight and 59% obese – yet they were notably slim sixty years ago when still eating their traditional diets. [1]
In our book, we note that the traditional diets of Pacific Islanders are almost toxin-free. A logical inference is that because they have for millennia eaten the world’s least toxic diets, Pacific Islanders never needed to evolve (or lost) an ability to cope with toxin-rich diets, and now suffer much more harm from toxic foods than do peoples whose ancestors have eaten toxic diets.
Age-Based Differences in the Biological Response to Unhealthy Food
It’s also the case that we respond to food differently as we age.
It’s not only digestion, such as the age-related decline in lactase enzyme expression, that changes. There are metabolic changes.
The elderly consume far fewer calories than the young; presumably evolution selected for minimal food utilization so that they would not be a burden to those who had to hunt and gather on their behalf. Their contribution was likely cultural, which didn’t require extensive physical activity.
Another change is that the elderly become less likely than the young to store calories in adipose tissue. This has significant consequences.
We know from a broad range of evidence that adipose tissue protects other tissues from damage by lipotoxicity; and that when adipose tissue refuses to store fat, obesogenic diets lead to metabolic syndrome and diabetes. [2] So reduced storage of calories in adipose tissue in the elderly will lead to (a) reduced rates of obesity (as measured by adipose tissue accumulation), but (b) higher rates of metabolic syndrome and diabetes.
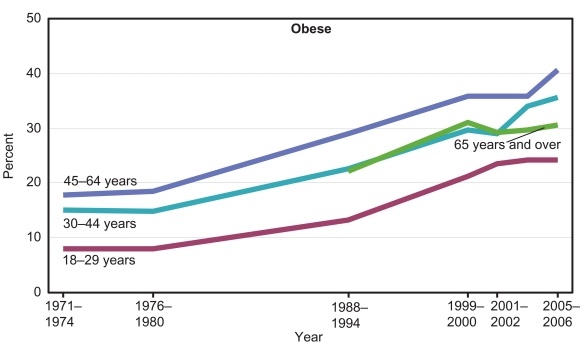
This is exactly what we see. Here are obesity rates by age group [3]:
Obesity rates for people over age 65 are lower than for people aged 30-64.
Here are diabetes rates by age group [4]:
Despite their lower obesity rates, the elderly have higher diabetes incidence.
This difference alone is sufficient to answer the question in our title: Yes, the elderly do need a Paleo (ie healthy) diet more than the young. Diabetes is much more dangerous than adipose tissue accumulation, so the elderly will suffer greater health impairment from an obesogenic (and diabetes-genic) diet than the young.
Is There Data Specifically Testing Rose’s Idea?
Rose’s idea that an evolved tolerance for toxin-rich diets will be specific to reproductively-aged persons with agriculturalist ancestors, is, so far as I know, not easily tested by available empirical evidence.
Studies in western populations alone will not be able to test Rose’s idea, because greater intolerance of toxic diets with higher age could simply be a result of an aging process that is universal in all populations. In order to find a process that recently evolved in agriculturalists, we would have to look at rates of aging or morbidity in different populations, both western and aboriginal, and see how aging rates or disease incidence depend on dietary toxicity:
- Are Pacific Islanders more likely than westerners on similar diets to develop diabetes at reproductive ages, but equally likely at late ages? Are they more likely to become obese at younger ages than old?
- Is aging more rapid in traditional peoples than in westerners during reproductive years, but similarly fast during elderly years, if they eat similar diets?
I am not aware of any such studies. Let me know if you are!
References
[1] Cassels S. Overweight in the Pacific: links between foreign dependence, global food trade, and obesity in the Federated States of Micronesia. Global Health. 2006 Jul 11;2:10. http://pmid.us/16834782.
[2] Unger RH, Scherer PE. Gluttony, sloth and the metabolic syndrome: a roadmap to lipotoxicity. Trends Endocrinol Metab. 2010 Jun;21(6):345-52. http://pmid.us/20223680. Sun K et al. Adipose tissue remodeling and obesity. J Clin Invest. 2011 Jun;121(6):2094-101. http://pmid.us/21633177.
[3] Health, United States, 2008: With Special Feature on the Health of Young Adults. National Center for Health Statistics (US). http://www.ncbi.nlm.nih.gov/books/NBK19623/.
[4] 2011 National Diabetes Fact Sheet, http://www.cdc.gov/diabetes/pubs/estimates11.htm.













Recent Comments