UPDATE: This was cross-posted on Jimmy’s site, so discussion is occurring on both sites.
I’d like to thank Jimmy for organizing this discussion on the desirability of including starches in a low-carb diet. (See: Is There Any Such Thing as “Safe Starches” on a Low-Carb Diet?). Not many people could bring such a roundtable together, and it’s an honor for us to be part of it.
I think unfortunately the discussion began with a few misunderstandings. So let me start with a few clarifications:
- We advocate a low-carb diet. “Low-carb” to us means eating less than the body’s actual glucose utilization, so that a glucose deficit has to be made up by gluconeogenesis.
- The concept of “safe starches” has nothing to do with their glucose content. “Safe starch” is a term of our invention and refers to any starchy food which, after normal cooking, lacks toxins, chiefly protein toxins. We do not consider glucose to be a toxin, though it may become toxic in hyperglycemia. Thus wheat, which includes gluten and various inhibitors of digestion that survive cooking, is an unsafe starch, while white rice, in which the known toxins (possibly excepting a recently discovered miRNA) are destroyed in cooking, is a safe starch. To say that something is a “safe starch” is not to imply that it is a desirable food for, say, a Type I diabetic.
- Our “regular” diet is not specifically directed at diabetic or metabolically damaged persons. We have a basic diet that is designed for healthy people (represented in the apple – food plate) and we recommend modified versions of the diet for various health conditions – including diabetes.
- We agree that diseases of metabolic derangement may benefit from lower carb consumption than our regular diet. This is especially the case in diabetes, if beta cell loss has reduced basal insulin levels and excessive gluconeogenesis is occurring. In this case, replacing protein rather than providing dietary carbs may be a more helpful strategy.
- We agree that there is no single prescription that is optimal for every person. We often say, borrowing from Tolstoy, that “every healthy person is biologically alike, every diseased person is unhealthy in his own way.” Obesity, for instance, is a heterogeneous disease and there is not a single prescription that will be optimal for every obese person. Diseases of metabolic derangement raise rather complex issues which we explore regularly on our blog. The science remains somewhat unsettled. But we do favor a low-carb approach.
After reading all the responses, it seems to me the debate boils down to two primary questions:
- On low-carb diets, is it better to eat 400 carb calories per day, as we argue, or some lower number of carb calories, say 100 calories per day?
- Are “safe starches” the best source of carb calories?
After answering these I’ll respond to individuals.
Part I: Why Are 400 Carb Calories Better Than 100?
This is a pivotal claim of our diet and apparently the core issue of debate. Allow me to discuss the biology in some detail.
Glucose Utilization of the Human Body
Brain and nerves typically consume about 480 calories per day of glucose. Ketones can displace up to perhaps 60% of this, but ketones do not diffuse well into cortical areas of the brain and the brain always requires some glucose.
After 3 days of fasting, when the brain’s glucose consumption has been roughly halved by ketosis and the rest of the body is conserving glucose, the body’s rate of glucose manufacture in liver and kidneys is about 600 calories per day. [1]
Two things to note:
- Even in fasting, peripheral utilization of glucose exceeds the brain’s.
- The fasting level of glucose utilization is likely to be suboptimal for health: fasting invokes glucose-and-protein-conservation measures which evolved to make us more likely to survive famine, but almost certainly have a cost in long-term health. (The logic is similar to Bruce Ames’s triage theory [2].)
This fasting level of glucose production of about 600 calories per day is a key number: the body must obtain glucose at at least this level, either through diet or endogenous production, if it is to avoid a glucose deficiency.
When not fasting, the body’s glucose utilization is somewhat higher – say, 800 to 1000 calories per day for a sedentary person. Glucose needs are slightly reduced by some endogenous sources of glucose, such as from glycerol released from lipolysis of triglycerides or phospholipids. So the body’s net glucose needs are on the order of 600 to 800 calories per day.
As I noted above, we consider Perfect Health Diet to be a low-carb diet because we favor eating fewer carbs than the body utilizes. For most people, we suggest 400 to 600 carb calories per day, about 200 less than the body utilizes. The remainder is made up by gluconeogenesis – manufacture of glucose from protein. We are a slightly or moderately low-carb diet.
The Human Glycome
Why is so much glucose consumed outside the brain? Immune function (which may utilize significant glucose in people with infections) and glycogen replacement (high utilization in athletes) are two reasons that can be significant in some persons, but in the vast majority of people the biggest reason for glucose utilization is the construction and maintenance of the human glycome.
There are about 20,000 human genes and, due to transcriptional variants and manufacture of proteins from multi-gene subunits, about 200,000 human proteins. However, these proteins are subject to various post-translational modifications, chief of which is glycosylation. Over half of all human proteins need to be glycosylated for proper function, and such is the variety of ways in which they can be glycosylated that there are an estimated 2,000,000 compounds in the human glycome.
These glycosylated proteins coat the plasma membrane of all cells. For many proteins, only glycosylated forms are allowed to leave the endoplasmic reticulum and Golgi complexes where they are formed; nonglycosylated forms are ubiquinated and destroyed.
Nearly every major extracellular molecule has significant carbohydrate content. Glycosaminoglycans such as hyaluronan and proteoglycan components such as heparan sulfate and chondroitin sulfate are important building blocks of the extracellular matrix. Proteoglycans in general mediate all intercellular interactions.
All the body’s lubricating molecules are rich in carbohydrate. Mucins, the most important molecules in mucus, tears, and saliva, are predominantly composed of carbohydrate. Mucin-2, the dominant mucin of the intestine, is 80% sugar by weight.
Production of hyaluronan alone consumes 5 gm, or 20 calories, of glucose per day. [3] I have been unable to find detailed measurements of daily mucin production, but if mucin constitutes 1.5% of the 400 g daily stool weight, then it consumes 5 gm of glucose per day. Since gut flora can break down and metabolize mucin sugars, this may be an underestimate.
So: whole body measurements indicate peripheral glucose utilization of around 100 to 150 g (400 to 600 calories) per day in normal humans, and a mere two of the 2,000,000 carbohydrate-containing compounds in the human body account for nearly 10% of that.
Glucose Deficiency Symptoms
Several responders argued that there cannot be such a thing as a human glucose deficiency on very low-carb diets because blood sugar levels do not leave the normal range.
However, this argument may prove a bit too much, because blood sugar levels don’t leave the normal range during human starvation either, and yet it still proves fatal. Why, if cells can run on glucose and blood glucose remains normal, do starving people die?
A clue is the fact that starving people develop a hacking cough in their final weeks of life. Despite blood glucose levels in the normal range, they cease producing mucus and their airways become dry and irritated.
The reality is this: peripheral glucose utilization is not determined by blood glucose levels, but is hormonally regulated. The brain may import glucose passively, driven by a concentration gradient, but not so the rest of the body. During times of glucose scarcity, blood glucose levels are maintained to sustain brain and nerve function, but hormonal patterns change to prevent peripheral tissues from using glucose to make compounds like hyaluronan and mucin.
What are the hormones that regulate glucose utilization? This is an understudied area of physiology, but the primary regulators seem to be thyroid hormones. During glucose deficiency, T3 thyroid hormone levels decrease and reverse-T3 levels increase. I discussed this in a recent blog post (Carbohydrates and the Thyroid, Aug 24, 2011).
Decreased production of molecules like hyaluronan and mucin and reduced levels of T3 thyroid hormone, then, are outcome of dietary glucose deficiency. Pathologies this may produce include dry eyes, dry mouth, constipation or hard stools, and slow healing of scratch wounds.
Do Glucose Deficiency Symptoms Actually Occur in Low-Carb Dieters?
Yes.
I discussed the reduced mucus production very low-carb dieters sometimes experience in an early blog post (Dangers of Zero-Carb Diets, II: Mucus Deficiency and Gastrointestinal Cancers, Nov 15, 2010). Since that was published, well over 50 low-carb Paleo dieters have reported to me that dry eyes and other mucin deficiency symptoms were cured by adding safe starches to their diet.
I have put up a “Results” page which has case studies drawn mainly from the comment section of my blog. This includes many cases of glucose deficiency symptoms that developed on very low-carb Paleo or GAPS diets and were cured on our diet. (GAPS is a very low-carb diet.) Here is a sampling.
Angie:
All four people in my family experienced a variety of new symptoms (seasonal allergies, constipation, worsening of heartburn, bladder spasms, dry eyes, increasing tiredness and low energy) when we did GAPS. These problems didn’t resolve until we luckily stumbled upon PHD and added back safe starches.
Susan:
I’ve instituted “Paleo” in our house since 1/1/11. Very strict about only plants and protein. About 4/1/11 I realized I was experiencing extremely dry eyes and mouth. I read your post about glucose deficiency and added rice and potatoes back into our diet. This cleared the problem up within 3 days and I was super grateful.
Melinda:
I had severe dry eyes while eating too low carb. Following Dr. Paul’s recommendations at “Perfect Health Diet”, I upped my carbs to his minimum of 50 grams of starch per day and the dry eyes went away.
There are many more cases; in addition to those on my “Results” page, many anecdotes can be found on PaleoHacks and in my comment thread.
What is the incidence of such deficiency symptoms on low-carb diets? At the Ancestral Health Symposium, two dozen people came up to Shou-Ching and I and told us their health had been improved by adding safe starches to their low-carb Paleo diets. As this was about 5% of conference attendance of 500, and not all people at the conference were low-carb and only a minority had tried our diet, I think it’s a safe bet that at least 20% of people who eat very low-carb diets will experience overt glucose deficiency symptoms.
Another Low-Carb Risk: Impaired Immunity
Low-carb diets generally improve immunity to bacteria and viruses, but not all is roses and gingerbread.
Low-carb diets, alas, impair immunity to fungal and protozoal infections. The immune defense against these infections is glucose-dependent (as it relies on production of reactive oxygen species using glucose) and thyroid hormone-dependent (as thyroid hormone drives not only glucose availability, but also the availability of iodine for the myeloperoxidase pathway). Thus, anti-fungal immunity is downregulated on very low-carb diets.
Moreover, eukaryotic pathogens such as fungi and protozoa can metabolize ketones. Thus, a ketogenic diet promotes growth and systemic invasion of these pathogens.
As the fungal infection case studies on our “Results” page illustrate, low-carb dieters often develop fungal infections, and these often go away with increased starch consumption.
Another issue is that mucus is essential for immunity at epithelial surfaces, and glycosylation is essential for the integrity of cellular junctions and tissue barriers such as the intestinal and blood-brain barriers. Thus, reduced production of mucus can impair intestinal immunity and promote gut dysbiosis or systemic infection by pathogens that enter through the gut.
Finally, a very low-carb diet is not entirely free of risks of gut dysbiosis, and not just from fungal infections. Bacteria can metabolize the amino acid glutamine as well as mucosal sugars, so it is not possible to completely starve gut bacteria with a low-carb diet. Nor is it desirable, as this would eliminate a protective layer against systemic infection by pathogens that enter the body through the gut. As our “Results” page shows, several people who had gut trouble on the very low-carb (and generally excellent) GAPS diet were cured on our diet.
The Possibility of Slow-Developing Problems Cannot Be Ruled Out
The majority of very low-carb dieters may experience no immediate ill effects. However, this does not guarantee that problems cannot develop over time.
Is it possible that peripheral downregulation of glucose utilization may increase the risk of some chronic diseases? There is too little experience with very low-carb diets to answer this question, but I think no biomedical scientist would exclude the possibility.
Biomedical researchers are gradually realizing the importance of glycosylation defects in leading diseases. I’ve mentioned previously that downregulation of glycosylation is an important part of the cancer phenotype (see An Anti-Cancer Diet, Sep 28, 2011; Dangers of Zero-Carb Diets, II: Mucus Deficiency and Gastrointestinal Cancers, Nov 15, 2010). A few papers:
- N- and O-glycosylation of proteins in Golgi bodies is impaired in cancer cells. [4]
- Cancer cells have systematically incomplete glycosylation, including deficient galactosylation of terminal beta-N-acetyl-D-glucosamine residues. [5]
- Genetic defects in O-glycans production increase cancer susceptibility. [6]
A recent report in Nature Medicine found that a specific glycosylation defect may commonly underly Type 2 diabetes. [7] From the abstract:
[A] deficit of GnT-4a glycosyltransferase expression in beta cells … produced signs of metabolic disease, including hyperglycemia, impaired glucose tolerance, hyperinsulinemia, hepatic steatosis and diminished insulin action in muscle and adipose tissues. Protection from disease was conferred by enforced beta cell-specific GnT-4a protein glycosylation and involved the maintenance of glucose transporter expression and the preservation of glucose transport. We observed that this pathogenic process was active in human islet cells obtained from donors with type 2 diabetes … [7]
I report these papers, not because I think they tell us how many carbohydrates we should eat – they don’t – but to remind everyone of the complexity of biology.
We lack data on the long-term effects of very low-carb diets. On the Standard American Diet, many diet-induced diseases do not show up for 40 to 50 years. Very low-carb diets have become popular only in the last few years. We cannot be sure that there may not be negative health effects from severe carb restriction that will show up only after decades.
I am not saying that such insidious health effects exist. I am only saying that while I believe low-carb is good, I don’t believe that very low-carb is better, and I think everyone should acknowledge that very low-carb diets may have unexplored risks.
In conclusion: Moderation in carb-hostility is no vice.
Part 2: Are “Safe Starches” Healthy Carb Sources?
So far I’ve defended our recommendation of 400 carb calories per day. Now we reach the question of which plant foods should provide them.
The main choice is between starchy plants and sugary plants. Sugary plant foods typically provide a mix of glucose and fructose; starches digest entirely to glucose.
Loren Cordain, whose “Paleo Diet” recommends a carb intake similar to or larger than ours, favors sugary plants:
[A]nyone who advocates eating white rice and potatoes obviously is unaware of the concept of either glycemic index or glycemic load … Yams, sweet potatoes, plantains and berries are healthful carb sources that most people can eat without a problem.
Yams, sweet potatoes, plantains and berries – all, by the way, foods our diet recommends and that we eat ourselves – contain some sugars which digest to a mix of glucose and fructose, while rice and potatoes contain starches which digest to glucose alone.
Because the concepts of “glycemic index” and “glycemic load” refer to blood glucose levels, they are sensitive to the glucose content of food, not the fructose content. Pure fructose has a glycemic index of only 19, compared to 100 for glucose.
We favor starchy plants over sugary plants for several reasons:
- Nutritional value. Glucose is more nutritious because, as noted above, it has structural uses throughout the human body. Fructose has no structural uses.
- Toxicity. Glucose is less toxic than fructose for several reasons. First, it is less reactive, less likely to glycate (fructate) proteins or promote lipid peroxidation. Second, Paracelsus’s rule tells us that the “dose makes the poison.” Dietary glucose is distributed via the blood throughout the body, so that levels are low in any one location. Fructose, however, is concentrated in the liver.
The body’s evolved machinery for handling glucose and fructose is a good indicator of their relative healthfulness. Glucose is treated by our evolved physiology as a non-toxic nutrient: it is allowed free entry to the blood where it is accessible to all cells of the body. Fructose is treated by our evolved physiology as a toxin: it is shunted to the liver where it is rapidly disposed of.
The toxicity of fructose is well supported by a host of biochemical, biomedical, and epidemiological data. In general, the more fructose people consume, the worse their health. Dr. Robert Lustig spoke at the Ancestral Health Symposium on this topic.
While I think glucose should be favored over fructose, I don’t want to exaggerate the dangers of limited fructose consumption: fruits, berries, and other sugary plants are, in moderation, fine components of a healthful diet. But I see no obvious reason to tout them as superior to starchy plants.
Glycemic Index and Load in Dietary Context
I do not believe that “glycemic index” or “glycemic load” are sufficient indexes of the healthfulness of foods.
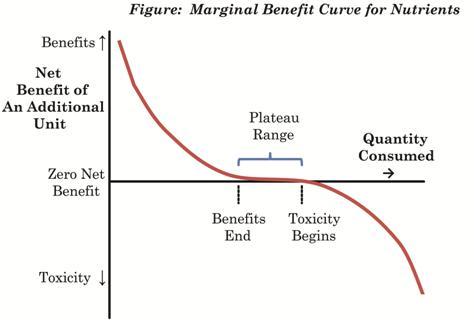
Glucose, as I’ve been arguing, is a nutrient: it has beneficial uses in the body. Nutrients generally deliver their greatest benefits when the body is deficient in them; few benefits when the body is replete; and often become toxic at high doses. Here is a figure from our book (p 4):
A “glycemic load” can be understood as a bolus of glucose delivered to the body. In a condition of glucose deficiency, a “glycemic load” is likely to be highly beneficial: it will be nourishing and repair the nutrient deficiency.
At higher levels of carb intake, a “glycemic load” is likely to be health-neutral – neither damaging nor beneficial. At very high carb intakes, a “glycemic load” may become dangerous.
So knowing a plant’s “glycemic index” or “glycemic load” cannot tell us whether it is good to eat some. That depends on the context of the rest of the diet. On a low carb diet, a safe starch is likely to be nourishing, regardless of its glycemic index.
Issues of Glycemic Control
In interpreting the safety of glucose, there is also the issue of whether postprandial increases in blood sugar can create transient toxicity effects. What is a dangerous level of blood glucose?
In diabetics, there seems to be no detectable health risk from glucose levels up to 140 mg/dl, but higher levels might have risks. Neurons seem to be the most sensitive cells to high glucose levels, and the severity of neuropathy in diabetes is correlated with how high blood glucose rises above 140 mg/dl in response to a glucose tolerance test. [8] In people not diagnosed with diabetes, there is also some evidence for risks above 140 mg/dl. [9]
For several reasons brief excursions above 140 mg/dl are probably not a problem for healthy people. However, for purposes of argument I’ll stipulate that a blood glucose level over 140 mg/dl probably does some mild harm.
Does eating a safe starch necessarily raise blood glucose above this level? No.
I offer as Exhibit A the experience of Haggus Lividus on Jimmy’s thread. Haggus measured blood glucose levels after consuming ~100 calories of rice and found that blood glucose levels peaked at 7.7 mmol/l = 139 mg/dl. Within an hour and fifteen minutes they were back at 5.8 mmol/l = 104 mg/dl. After sweet potatoes, blood glucose peaked at 6 mmol/l = 108 mg/dl.
These are safe levels of blood glucose – below 140 mg/dl at all times. Yet Haggus Lividus took these as levels to be unsafe!
Tom Naughton reports that a potato raises his blood glucose level to 175 mg/dl. This is, indeed, an unsafe blood glucose level.
But he eats a very low-carb diet, and very low-carb diets induce hormonal changes that lead to glucose conservation. One result of these changes is insulin resistance and impaired glucose tolerance.
Thus, an isolated glucose tolerance test is not necessarily a fair test of glycemic control in a very low-carb dieter. Were Tom to eat 400 calories per day from safe starches for a week, he might find his glycemic control was considerably improved. Or, he may find that he is somewhat diabetic and intolerant of carbs in all circumstances.
What is a normal blood glucose response to consumption of a starchy meal? Here is a view of blood glucose levels in normal people as measured by Professor JS Christiansen (from Ned Kock via CarbSane):
Although a majority maintain blood glucose levels below 140 mg/dl at all times, it is not unusual for blood glucose levels to enter the range 140 to 165 mg/dl for brief periods after meals. These measurements were all done in healthy young people.
Vegetables as Poor Glucose Sources
Some responders were understandably confused by a line Jimmy quoted out of context from our book: “don’t count vegetables as as a carb source – they are a fiber (and therefore a fat) source” (page 45).
The point is that vegetables are not usually helpful in repairing a glucose deficiency. A typical vegetable has about 80 carb calories per pound, half as glucose and half as fructose. The digestive tract typically consumes about 50 calories of glucose in digesting a pound of vegetable matter, due to intestinal and immune utilization. Some fructose may be converted to glycogen and then to glucose, but some may be converted to fat and much may be intercepted by gut bacteria. Fructose malabsorption is a widespread problem. So the net contribution of vegetables to the body’s glucose status is small and may be negative.
Since we recommend counting calories only for a few days until one learns how much one must eat to obtain our recommended 400 calories per day of glucose, there is no reason to include vegetables in calorie counting. Vegetables are recommended in our diet due to their micronutrient and fiber content, not their carbohydrate content.
Improved Weight Loss with Consumption of Safe Starches
Since many of Jimmy’s readers eat low-carb diets in the hope of losing weight. It may be of interest to them to know that some of our readers have experienced easier weight loss, reduced appetite, and diminished food cravings after adding “safe starches.” Our “Results” page has examples.
Part 3: Specific Replies
Readers may wish to open Jimmy’s post, Is There Any Such Thing as “Safe Starches” on a Low-Carb Diet?, in another window to follow along.
Colette Heimowitz does not seem familiar with our work, and to have misunderstood the basis for our recommendation of a modest amount of starch. We do not come from a “glucose mentality” and agree that fat and ketones are fine metabolic fuels. However, ketones do not eliminate glucose needs.
The fact that glucose can be formed via gluconeogenesis does not prevent the emergence of glucose deficiency conditions, because the degree of gluconeogenesis is hormonally controlled and may be insufficient to maintain all normal glucose functions.
Maintainance of blood sugar is not an indicator that there is no glucose deficiency.
Glycation is one thing, glycosylation and manufacture of GAGs and other glucose containing structural molecules of the human body is another. We agree that glycation is bad.
I’d like to thank Robb Wolf for his point of view, which is quite reasonable. I am not asserting that no one can do well on a very low-carb diet, only that as carb consumption approaches zero risks of health problems increase. That Robb himself experienced problems on sustained very low-carb is a helpful data point.
I’d like to thank Chris Masterjohn for his contribution. I think Chris has read enough of our work to know that we recommend ketogenic diets as a therapy for various conditions, including neurological disorders of all kinds, and generally hold that dietary adjustments are desirable in many health conditions. So we do not consider that a single macronutrient ratio applies to everyone, but we do believe that intolerance of a “normal” macronutrient ratio is diagnostic of a dysfunction of some kind.
Chris is quite right that it’s a “safe[r] bet” to meet the body’s physiological need for glucose in part by eating glucose. This reduces the risk of failing to provide adequate glucose for optimal cellular and extracellular function.
I’d like to thank Dr. Kurt Harris for his contribution. I discussed Dr. Harris’s post on my blog: https://perfecthealthdiet.com/?p=4802.
Dr Jonny Bowden makes an excellent point: a major advantage of starches over other carbohydrate sources is their lack of fructose. Glucose is, in general, a safer carb source than fructose.
Dr Robert Su directs us to an essay of his, which makes a lot of points that I agree with, but his evidence doesn’t imply the conclusion that all carbs should be excluded, nor does it address the main issues of our diet.
Tom Naughton and I share Irish ancestry, so if he has been extinguished due to lack of ancestral potatoes then so have I. Luckily for both of us, failure to consume safe starches, if that is what our ancestors did, is not so damaging to health as to necessarily result in early death and failure to leave descendants.
That his blood glucose rises to 175 mg/dl after consuming a potato indicates one of two things: his glucose regulation is irretrievably broken and he must never again eat a whole potato in isolation from other foods, or he is insulin resistant in order to conserve glucose and he should eat carbohydrates more often to improve his insulin sensitivity. Which is his optimal course is not something I can know.
Dr Richard Feinman may not have noticed but Shou-Ching and I were at the Ancestral Health Symposium and so were dozens of people following our diet; indeed, about two dozen people came up to us and told us that our diet had improved their health. The most frequently cited benefit was feeling better after adding safe starches to the diet, with relief of dry eyes the most common symptomatic improvement. So if symposium attendees were not dropping like flies, perhaps we deserve a bit of the credit.
Dr. Loren Cordain’s assertion that eating sugary plants like yams, sweet potatoes, and berries is preferable to eating starchy plants like rice and potatoes may be a defensible position, but we believe the evidence is strong that glucose is preferable to fructose as a carb source, and does not support the notion that rice or white potatoes are intrinsically dangerous foods.
Dana Carpender links to one of Mike Eades’s best posts, which we cite and quote in our book’s discussion of why wheat bran is unhealthy. However, it in no way rebuts our observations about the negative health effects of a deficiency of mucus arising from a glucose deficiency.
We agree with Dana that turnips, rutabaga, Jerusalem artichokes, and jicama are fine foods.
Anonymous Prominent Member makes a good point: adding carbs back into a very low-carb diet worked for me, but may not work for everyone. I agree with Anonymous Prominent Member’s point about the importance of practical experience. I think this is one of our greatest strengths. Thousands of copies of our book have been sold, and hundreds of people have reported results back to us. These reports have been overwhelmingly positive. On the blog, I answer questions from people with health problems, ask them to report back results, and many return weeks or months later to report cures or improvements. I invite Anonymous Prominent Member to review the case studies on our “Results” page.
Dr. Uffe Ravnskov can find the scientific studies in support of our views on our blog and in our book. Nowhere do we assert that it is impossible to survive on a zero-carb diet. Rather we assert that a zero-carb diet is suboptimal for health, and not robust to certain health problems, such as some infections.
I would like to thank Adele Hite for her generous statements that our “overall approach is very reasonable” and “may be useful to many people,” and for her engagement on issues of substance.
Adele links to Mike Eades’s excellent fiber post, which we cite approvingly in our book; see my comment to Dana Carpender. The issue Mike discussed, of an excess of mucus due to intestinal injury, is unrelated to the issue we discuss, of a mucus deficiency due to glucose deficiency.
About vitamin C, I think Jimmy may have given this issue quite a bit more prominence than it deserves. It happens that the incidence of kidney stones, glutathione deficiency, and vitamin C deficiency is increased on very low carb ketogenic diets for epilepsy, and other very low carb diets. I made a speculative post attempting to guess the causes of this. To answer Adele, part of the issue is likely a protein deficiency: the need to utilize protein for gluconeogenesis may induce a protein deficiency on an otherwise adequate dietary intake. Other factors are that vitamin C degrades through a pathway that generates oxalate in the kidneys, a risk factor for calcium oxalate stones. Vitamin C does indeed share insulin-dependent receptors with glucose, which implies that glucose competes with C but also that insulin promotes C entry into cells for recycling, so the overall effect of consuming carb-rich foods is unclear. On a low-carb diet adding a little dietary glucose is unlikely to be pro-inflammatory.
About cancer, this is an interesting scientific question. I’ve explained above why a glucose deficient diet can downregulate production of glycoproteins and other structural glucose-containing compounds. However, cancers often evolve an ability to take in glucose independently of insulin and other hormones that regulate glucose utilization in normal cells. As a result, one could argue that things would run the opposite way than Adele proposes: reducing dietary glucose, which generally does not reduce blood glucose levels, will not affect cancer metabolism, but will limit availability of glucose to normal cells for structural use.
I would like to thank Dr. Larry McCleary for addressing matters of scientific substance in a well-reasoned comment. He is quite right that cancers disable glycosylation by suppressing enzymes involved in it. Our reasoning, admittedly speculative, is that (a) the cancer cellular phenotype is a wayward phenotype characterized by reduced intercellular cooperation, cooperation that is largely mediated through glycoproteins, proteoglycans, and glycosylated proteins; (b) cells evolve the cancer phenotype in part by disabling the enzymes which glycosylate proteins; (c) therefore (the speculative inference) dietary steps which downregulate glycosylation may inadvertently serve to entrench or promote the cancer phenotype. This is speculative science, but speculation is the first step in scientific discovery.
Dr McCleary is quite right that depriving cancer cells of glucose is an attractive therapeutic strategy for cancer. However, except in the brain (where ketogenic dieting can significantly reduce glucose levels) this is a difficult strategy to implement. Blood glucose levels are maintained even through the late stages of starvation, and cancer cells can evolve insulin-independence and the ability to import glucose massively from blood. Paradoxically, eating some dietary carbs can even decrease average 24-hour blood glucose levels by increasing insulin sensitivity in normal cells.
I would like to thank Chris Kresser for an excellent comment sharing his clinical experience:
In cases where there is no significant metabolic damage, when I have these folks increase their carbohydrate intake (with starch like tubers and white rice, and fruit) to closer to 150g a day, they almost always feel better. Their hair loss stops, their body temperature increases and their mood and energy improves.
For people that are overweight and are insulin/leptin resistant, it’s a bit trickier. In some cases increasing carbohydrate intake moderately, to approximately 100g per day, actually re-starts the weight loss again. In other cases, any increase in carbohydrate intake – in any form – will cause weight gain and other unpleasant symptoms.
This corresponds precisely with our recommendations. Healthy people will do best on 100-150g per day; obesity is a heterogeneous disease and some will do best on a carb intake in that normal range, others (especially those who are more diabetic) will do best on very low-carb diets. Our “Results” page includes feedback from a number of people who lost weight on our diet better than on other low-carb diets.
I would like to thank Dr David Diamond for a thoughtful comment and for taking the time to read our blog. It is gratifying that he eats largely in accord with our recommendations and has had good results: “This has been my basic diet plan for the past 6 years and my blood lipids have responded in the right directions and I’ve lost about 25 lbs.”
Nowhere do we assert that slipping to 300-400 carb calories is dangerous; rather this is in our “safe range” of 200 to 600 carb calories per day and very close to our estimated optimum. However, I do think that for healthy people the potential harms from very low-carb are greater than the potential harms from excessive carb consumption, so it is perhaps safer to advise eating in the upper end of the range, since a large number of people will deviate from their target.
Dr. Diamond notes that “I haven’t actually seen adverse health outcomes for most people who eat 50-100 gm of carbs/day.” Interestingly, 50 g is sort of a magic number of carbs for many people: there are adverse health outcomes eating less than 50 g, but intake of 50 g or more tends to eliminate them. Our comment threads, and other sites such as PaleoHacks, are full of people who have reported this experience. So I would agree with Dr. Diamond’s statement, but argue that it supports our recommendation to eat at least 50 g of safe starches.
I’ve discussed the cancer issue elsewhere, but I appreciate Dr Diamond’s contribution.
Livin’ La Vida Low-Carb Reader’s carb intolerance is a difficult problem to deal with; I sympathize, and largely agree with what LLVLCR says. LLVLCR may wish to read my reply to Tom Naughton; I would say something similar in LLVLCR’s case. Low-carb is good, control of blood glucose is good, but it is not obvious that zero-carb is optimal.
I agree with Dr. Andreas Eenfeldt that those with diabetes and metabolic syndrome may do better with lower carb intake than is optimal for healthy people.
Dr. Eenfeldt may wish to visit our “Results” page to learn about the mucus deficiency issue on very low carb. It can generally be healed with the addition of 50 g starch to the diet; sometimes vitamin C supplementation is needed as well.
As noted elsewhere, blood glucose levels are not an indicator of the body’s glucose status, and will remain normal even when there is a serious glucose deficiency. Production of glycoproteins such as mucin is a much more sensitive indicator of whole-body glucose status.
Dr. Jeff Volek should be aware that if “there is no defined condition associated with not consuming carbs,” it may be because biomedical scientists have spent little to no time observing people who do not consume carbs. Dr Volek may consult our “Results” page for examples of people who have developed adverse health conditions from very low-carb dieting.
I’d like to thank Dr Jeffrey Gerber for sharing his very interesting clinical experience with cancer patients:
Patients who are ill such as cancer, post surgical, after the hospital are stressed and their basic metabolic rate is increased. In this situation I have found that there is an increased caloric demand. Patients require more calories from fat protein and carbs.
Cancer is a very complex disease and Dr. Gerber’s experience is a helpful reminder that knowledge of the Warburg effect, while helpful for understanding cancer, is not sufficient knowledge to design an anti-cancer diet.
Dr. Jack Kruse’s only substantive sentence is this: “I think avoiding anything that stimulates the IGF1 pathway is ‘smart’ based upon current knowledge and i think using a ketogenic diet is also prudent.”
The dominant dietary factors stimulating IGF-1 release are “protein and energy intake … and energy intake may be of greater importance.” Our diet is generally lower in protein than other low-carb diets, and as a nourishing diet with macronutrient intakes near the body’s utilization needs, it is highly effective at minimizing appetite and total energy intake, as perusal of our “Results” page will show.
Using a ketogenic diet is sometimes prudent. We recommend a ketogenic diet for many neurological disorders and brain cancers, and readers have used our version of the ketogenc diet to cure migraines and ameliorate genetic diseases such as Neurodegeneration with Brain Iron Accumulation (see our “Ketogenic Diet” category for more). We also recommend practices that introduce ketosis intermittently, such as daily intermittent fasting, to everyone as a good general health practice.
Since our diet minimizes IGF-1 and is frequently ketogenic, I would have expected Dr. Kruse to be more positive. Perhaps his reaction may have been just a reflex: more IGF1 reduction, more ketosis, more cowbell. Or perhaps he favors the ultimate in low-IGF1, high-ketosis diets: the Terri Schiavo diet.
Dr. Fred Pescatore should read our book. It is not true that 200 calories of starch will necessarily take a person out of ketosis. Consumption of medium chain triglycerides or coconut oil in conjunction with starches will trigger a mild ketosis, see Ketogenic Diets, I: Ways to Make a Diet Ketogenic, Feb 24, 2011.
Glycosylation of proteins occurs primarily intracellularly in the endoplasmic reticulum and Golgi bodies, not on cell membranes.
I thank Dr. Eric Westman for looking at our web site and trying to understand our diet. Hopefully this response will have made things clearer.
Peter Dobromylskyj of “Hyperlipid” has had a very busy year with a new daughter and new home, so I’m not in the least surprised that he hasn’t yet had time to read our book. I hope he will enjoy it when he does, as he is one of my favorite health writers.
The human glycome is much more than a lectin signaling system: it has a myriad of structural and functional roles, some of them discussed above.
Re “I can’t see glucose deficiency being a gut problem as this is the organ with the highest exposure to dietary glucose,” two factors which limit availability of dietary glucose to gut cells are (a) dietary glucose is absorbed in the small intestine but gut problems are most common in the colon where bacterial populations are highest, and (b) we are considering very low carb diets that provide little dietary glucose. A third factor to consider is that the gut, due to its mucin production, immune activity, and rapid turnover in cells and extracellular matrix, is a major consumer of glucose.
Cancer is an extremely complex and interesting disorder and I’ll be delighted to hear Peter’s ideas.
Peter makes an extremely important point: that minor dietary defects may take decades to reveal themselves. On the Standard American Diet, an unhealthy diet, it often takes 50 years for chronic diseases to appear. If there are problems with very low carb diets, we should not necessarily expect them to appear immediately.
Peter says “I don’t know,” but in truth we all don’t know: dietary science is complex and all of our positions are somewhat speculative. Thus humility is in order.
I thank Dr. William Davis for his assessment that our “diet seems a rational, workable program” and agree with him that diabetics will benefit from reducing starch consumption.
Valerie Berkowitz should be aware that we do recommend tomato consumption, but we do not consider it a “safe starch” because its calories are mainly in the form of sugars. Her other concerns are addressed above.
We agree with Diane Sanfilippo’s observations.
Dr William Yancy seems to be intelligent, reasonable, and unfamiliar with our diet. Perhaps the exposition above will help.
Dr Ann Childers links to an article in Discover magazine and avers that the humans of the Ice Age and the Inuit were “without cancer, diabetes, tooth decay, glutathione deficiency, vitamin C deficiency or gut dysbiosis.” These claims are unsupported. Nor is it the case that Ice Age humans ate zero-carb diets, nor any other humans who had access to starchy plants.
Dr Cate Shanahan makes two important points with which we wholeheartedly agree.
First is her observation that the protein quality of food, especially the presence of immuno-reactive proteins, is extremely important for health. Indeed, our “safe starches” are defined by their lack of these toxic or immunogenic proteins. We strongly agree with this point, and it is a centerpiece of our diet.
Second is the point that just because it is possible to manufacture glucose from protein does not mean that optimal amounts of glucose will actually be manufactured if none are eaten. It is well established that macrobiotic dieters, who eat low-fat diets, can develop lipid deficiencies, notwithstanding the fact that lipids can be manufactured from glucose. Something similar can happen on very low-carb diets, especially if dietary protein is insufficient.
Amy Kubal seems to be under the misimpression that I recommend 1 pound of safe starches daily for “everyone.” No, this is a recommendation for healthy people, I understand that some people with defects of metabolic regulation or neurological disorders will benefit from ketogenic diets or severe carb restriction.
I agree with her advice about the benefits of carbs following workouts. The reason we recommend not counting carb calories from vegetables was discussed above.
It is not obvious to me from her description that her recommended cancer diet differs much from ours.
Dr Robert Su refers us to a column of his. It makes a lot of points whose truth I acknowledge, but doesn’t address any of the arguments I’ve made, and certainly doesn’t support the conclusion that there is no benefit from dietary carbohydrate.
I applaud Mark Sisson’s comment. Primal and Perfect Health Diet are indeed extremely close, and Mark properly focuses on the important points, such as avoiding grains, fructose, and seed oils. Mark’s easygoing attitude toward unimportant differences is praiseworthy.
Dr Lauren Noel notes that other than a few minor cell types, “all tissues can run on ketones,” and supposes this refutes the need for dietary carbohydrate. However, although the brain can run on ketones, it turns out that ketones don’t diffuse well to the cortical areas of the brain, and the brain always requires some glucose even in extreme ketosis. Also, while ketones can replace glucose as a fuel, they cannot glycosylate proteins, or generate ROS in the manner needed by immune cells.
Dr Noel believes that eating white rice and sweet potatoes will aggravate Candida infections. Dietary carbs can feed Candida in the gut, but they also feed competing probiotic bacteria and promote intestinal barrier integrity and immune function, and thus their effect on the gut flora is complex. More importantly, ketosis promotes systemic invasion by Candida and glucose is needed for the immune defense to Candida, so a moderate carb intake is helpful to the defense against systemic Candida. As Candida is an effective intracellular pathogen that can flourish systemically, this is a very important consideration. No one with a Candida infection should eat a ketogenic diet. Dr Noel might wish to consult our “Results” page for a few cases in which fungal infections were exacerbated on very low-carb diets and cured on our diet.
Dr Daniel Chong is quite right that starches have been a part of the evolutionary human diet, since at least Australopithecus 3.5 million years ago. The history may go back even farther: recent anthropology speculates that the common human-chimp ancestor may have been bipedal and lived in open woodlands where starches but not sugary fruits were the predominant food.
Dr Greg Ellis is rather quick to assert that our work is “made up” and “constructed out of thin air” even though he acknowledges not having read our book, and is under the misimpression that we have “bought into the dangers of fat and cholesterol.” He asserts, “If you want to talk about toxins then glucose is at the top of the list” which is absurd; among sugars alone, fructose is more toxic than glucose. He asserts, “If glycosylation is truly important there is enough glucose available to perform this function without eating glucose or carbs” which is precisely the point at issue. He blames cancer on glycation of proteins, a highly dubious claim. He is unaware that fungi are eukaryotic organisms that have mitochondria.
Dr Ron Rosedale has written an extended commentary which deserves a considered response. Since he posted a series on Facebook to which I had already begun drafting a reply, I’ll finish that and post it on my blog next week. I thank Dr Rosedale for the time he’s given to this discussion.
Dr Joe Leonardi’s comments are intelligent, and it sounds as though his own dietary advice is excellent. I thank him for his contribution.
Dr BG makes an excellent point: that carbs do in practice improve the health of many paleo dieters, in part via improving adrenal function. Dr BG herself reports, “I feel ‘better’ on higher carbs for the adrenals.” Dr BG also notes, “On Paleohacks there are countless stories of people on VLC paleo who feel dizzy or lightheaded. H-E-L-L-O this is cardinal signs and symptoms of adrenal fatigue. Many of these folks are also doing HIIT and hard core CROSSFIT!” Of course, exercise utilizes glucose and will exacerbate any glucose deficiency.
These cases of improved health upon higher carb consumption should be a warning to those other writers who question whether it’s possible to have a glucose deficiency.
Zoe Harcombe appears to approach dietary science from premises similar to ours. She shares our nutrient-based view and general orientation.
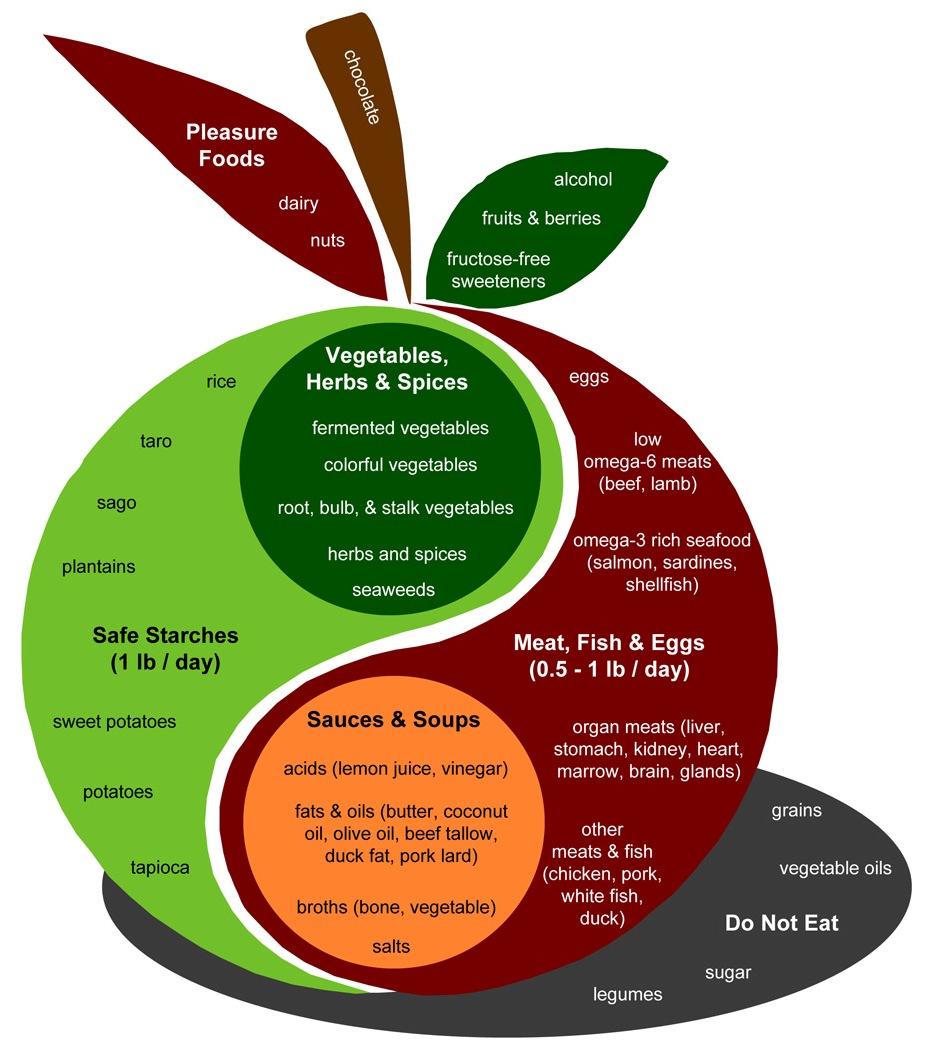
On the issue of taste, we do recommend that starches be eaten as part of a meal in combination with sauces, vegetables, fats, and meats. So yes, rice will often be combined with curry. In our “Food Plate,” the body of the apple signifies foods that are best eaten as part of a meal – starches, vegetables, meats, soups, sauce – and the “pleasure foods” are good snacks or desserts.
Our bodies do need glucose, and it may be preferable to obtain it directly from diet than to have to manufacture it from protein.
An appropriate population of commensal bacteria tends to stabilize the gut and make it resistant to dysbiosis. Antibiotics, starvation of carbohydrates, and other factors that deplete gut bacteria may increase the risk of fungal or other infections.
We do recommend lower carb consumption for diabetics.
The amount of glucose in blood is not related to the amount of glucose the body consumes in a day. The “stock” of glucose in blood is continually replenished by a “flow” from the liver as tissues draw it down. It is the flow, integrated over 24 hours, which is the daily glucose consumption.
The Taubesian idea of intentionally creating a glucose deficiency to force the body to breakdown triglycerides for glycerol is a clever but flawed strategy for weight loss. Its chief defect is that triglycerides break down to about 11% glucose by calories, but the body’s glucose utilization is close to 30% of energy. As a result, this strategy cannot meet glucose needs without releasing free fatty acids beyond energy needs. If these are not successfully disposed of, then blood free fatty acid levels may become elevated, which leads to the phenomenon of “lipotoxicity” which can promote diabetes. Whether and to what degree glucose deficiency and lipotoxicity would occur in any attempt to execute such a strategy is an empirical matter, but no reader should assume that such a strategy is riskless.
There is room to disagree about the optimal level of glucose intake, and I hope Zoe will look into our arguments for a slightly higher carb consumption than she is used to.
Dr Stephen Phinney seems to be under the misimpression that my term “safe starches” refers to low glycemic index foods. No, it has nothing to do with the carbohydrate; “safe” means that after cooking the food lacks toxic, bioactive, or immunogenic proteins. It is about the plant proteins, not the carbs.
Dr Phinney avers that “there is no absolute human requirement for dietary carbohydrate.” I am not sure what “absolute” means, but I do believe that health will usually be improved if the diet includes some carbohydrate.
Dr. Phinney defends his statement by reference to blood sugar levels. As discussed above, blood glucose levels are not an adequate indicator of the body’s glucose status.
Re the issue of vitamin deficiencies, there are plenty of reports of nutrient deficiencies on clinical ketogenic diets, thus Dr Phinney’s need to include the adjective “well-formulated” before ketogenic diets. I agree with him on this point: it is possible to formulate ketogenic diets in such a way that they don’t generate nutrient deficiencies. However, it is perilously easy to misformulate them. Diets should be robust to error. When carbohydrate intake approaches zero, diets become less robust. Since few people know how to properly formulate a ketogenic diet, this has to be considered a risk to low carb diets.
On the issue of dysbiosis, I assume Dr Phinney will agree that some non-zero level of mucus production is optimal, and that a level of mucus production below that optimum impairs health.
Dr Richard Bernstein is the author of a book we frequently recommend to diabetics, so it’s unfortunate that he may have gotten the mistaken impression we recommend higher carbohydrate consumption for diabetics. Perhaps he’ll look more closely into our diet and reconsider his judgment.
References
[1] Nair KS et al. Leucine, glucose, and energy metabolism after 3 days of fasting in healthy human subjects. Am J Clin Nutr. 1987 Oct;46(4):557-62. http://pmid.us/3661473.
[2] McCann JC, Ames BN. Adaptive dysfunction of selenoproteins from the perspective of the triage theory: why modest selenium deficiency may increase risk of diseases of aging. FASEB J. 2011 Jun;25(6):1793-814. http://pmid.us/21402715. McCann JC, Ames BN. Vitamin K, an example of triage theory: is micronutrient inadequacy linked to diseases of aging? Am J Clin Nutr. 2009 Oct;90(4):889-907. http://pmid.us/19692494.
[3] Stern R. Hyaluronan catabolism: a new metabolic pathway. Eur J Cell Biol. 2004 Aug;83(7):317-25. http://pmid.us/15503855.
[4] Hassinen A et al. Functional organization of the Golgi N- and O-glycosylation pathways involves pH-dependent complex formation that is impaired in cancer cells. J Biol Chem. 2011 Sep 12. [Epub ahead of print] http://pmid.us/21911486.
[5] Satomaa T et al. Analysis of the human cancer glycome identifies a novel group of tumor-associated N-acetylglucosamine glycan antigens. Cancer Res. 2009 Jul 15;69(14):5811-9. http://pmid.us/19584298.
[6] An G et al. Increased susceptibility to colitis and colorectal tumors in mice lacking core 3-derived O-glycans. J Exp Med. 2007 Jun 11;204(6):1417-29. http://pmid.us/17517967.
[7] Ohtsubo K et al. Pathway to diabetes through attenuation of pancreatic beta cell glycosylation and glucose transport. Nat Med. 2011 Aug 14;17(9):1067-75. http://pmid.us/21841783.
[8] Singleton JR et al. Increased prevalence of impaired glucose tolerance in patients with painful sensory neuropathy. Diabetes Care. 2001 Aug;24(8):1448-53. http://pmid.us/11473085. Hat tip Jenny Ruhl, http://www.phlaunt.com/diabetes/14045678.php.
[9] Ziegler D et al. Prevalence of polyneuropathy in pre-diabetes and diabetes is associated with abdominal obesity and macroangiopathy: the MONICA/KORA Augsburg Surveys S2 and S3. Diabetes Care. 2008 Mar;31(3):464-9. http://pmid.us/18039804.











Recent Comments