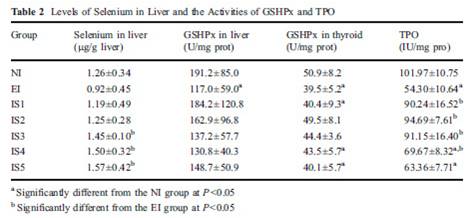
Robert Evans once questioned whether crème bruelle (which I assume is another name for crème brûlée) was PHD-compliant.
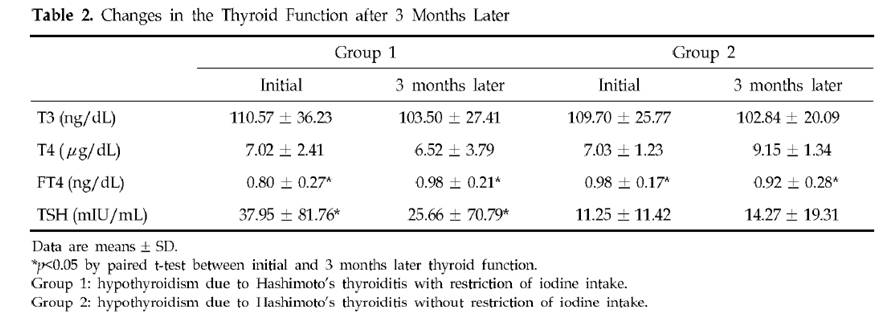
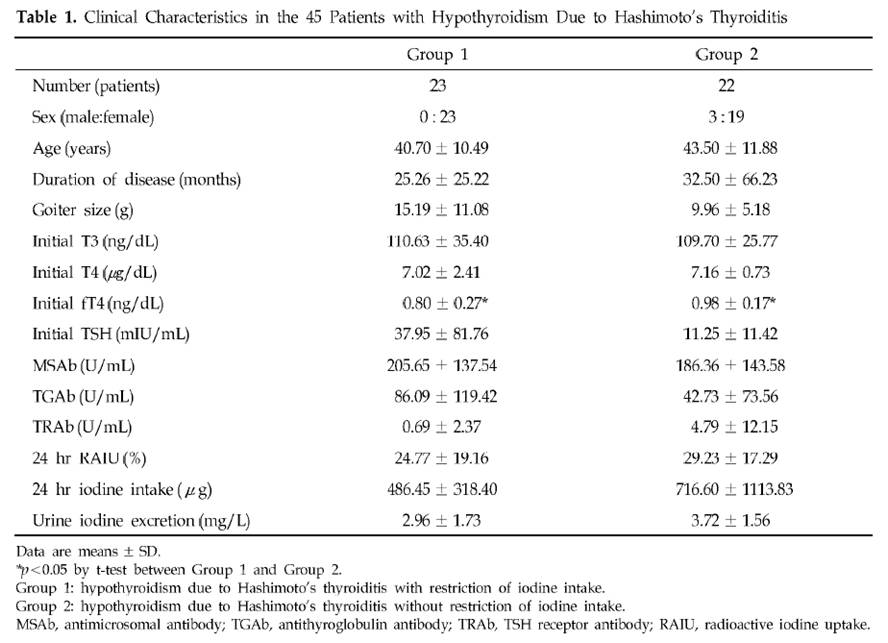
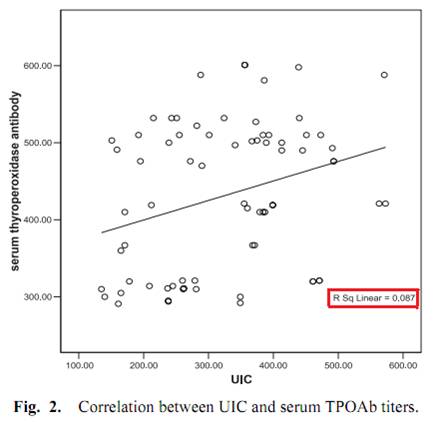
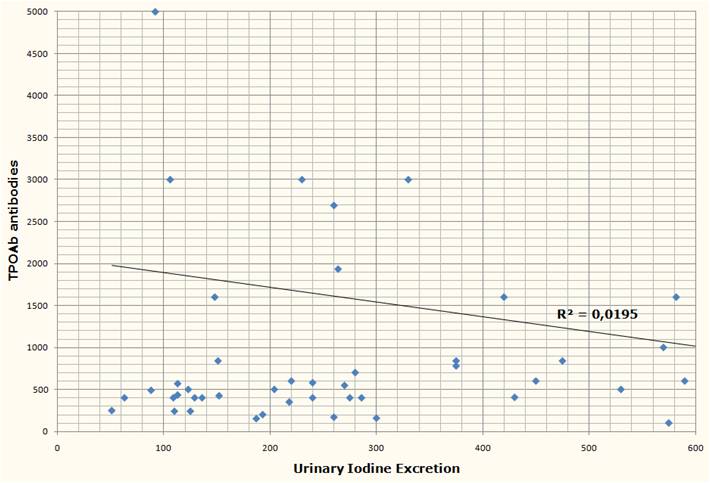
It is, and we decided to make some. It turns out it’s real easy.
Ingredients
Here’s what we used: ¼ cup rice syrup, orange zest, 1 cup heavy cream, 4 egg yolks, and about 5 drops vanilla extract. The orange zest is optional; lemon juice or a bit of lemon zest could also be used.
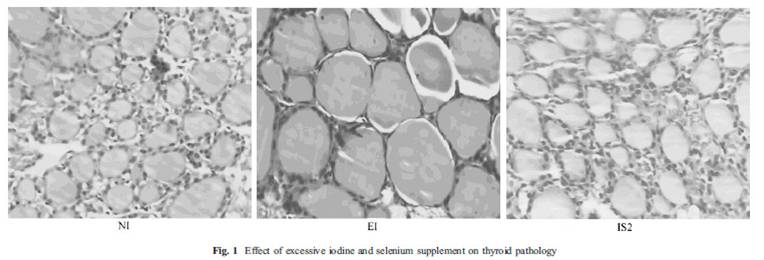
Cooking
Preheat the oven to 325 F (160 C).
Combine heavy cream, rice syrup, orange zest, and vanilla extract in a sauce pan and warm it at low heat for 5 minutes or so until it is tiny bubbles appear but it is not boiling.
While the cream mixture is warming, whisk the egg yolk.
Transfer the cream mixture a little bit at a time into the egg yolk and continue whisking, until all of the cream has been transferred.
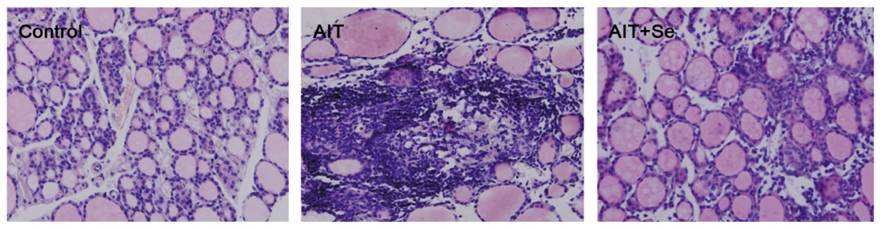
Then distribute the mixture into smaller containers suitable for serving, and place the small containers into a few inches of near-boiling water in an oven-safe pan:
Place the whole pan into the oven for 25 minutes at 325 F. After 20-25 minutes take a small container out and shake it; if the custard is firm then it’s done.
Remove the small containers and refrigerate them for at least 2 hours. We refrigerated them in this aluminum pan:
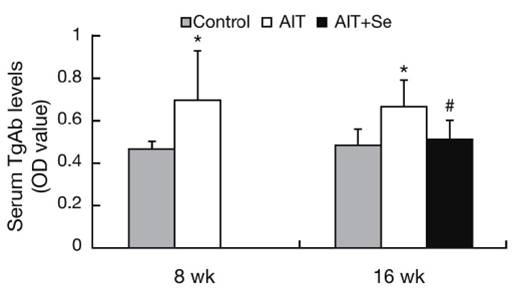
Once they are cold, the traditional recipe calls for sprinkling sugar on top and then caramelizing it with a blowtorch (or a broiler if the blowtorch is lacking).
Well, we didn’t have a blowtorch and didn’t feel obliged to caramelize our rice syrup, so we simply drizzled our custard with rice syrup or sprinkled it with cocoa powder:
It was delicious! Served cold, it’s great for summertime.






















Recent Comments