Bret asked us how much chocolate is needed for good health:
I have a question about having dark chocolate daily. Does it need to be every day or what is the mininum grams per day. I have been having around 35g a day of 70% but I wondered if less would be ok or not having it at all.
This is a great time for this question, since Halloween candy will be running out soon, and those on tight budgets may be tempted to skimp on their chocolate. Should they?
Chocolate Is Not Considered Essential … Yet
Chocolate has not yet been recognized by the Food and Nutrition Board of the National Academies as an essential nutrient. We haven’t either: Our food plate lists it among “pleasure foods,” which are healthful but optional.
However, we are becoming ever-more chocolate friendly. In the new edition of our book, we list chocolate among our “supplemental foods” which we recommend consuming regularly. But our suggested dose is “as desired.” Perhaps we should narrow that down a little.
Chocolate Against Cardiovascular Disease
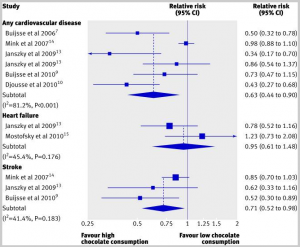
We’ve previously warned of the danger of chocolate deficiency, based on a systematic review that found: “The highest levels of chocolate consumption were associated with a 37% reduction in cardiovascular disease and a 29% reduction in stroke.” [1]
Here’s a visual summary of their findings:
The review authors report that every study accounted for chocolate intake in a different way, so they could only compare the groups with highest and lowest chocolate consumption in each study, not specific doses of chocolate.
Chocolate Against Diabetes
Bret was concerned about the sugar in chocolate, but if this is a problem, it’s outweighed by the benefits of chocolate. A Japanese study found that the rate of diabetes was reduced by 30% in those who consume the most chocolate. [2]
Chocolate Against Dementia and In Support of Cognitive Function
Several studies [3, 4] have found that chocolate consumption reduces risk of dementia and enhances performance on tests of cognitive function.
One of them found that cognitive function was optimized with a relatively low dose of chocolate – ten grams per day:
The associations between intake of these foodstuffs and cognition were dose dependent, with maximum effect at intakes of approximately 10 g/d for chocolate and approximately 75-100 mL/d for wine, but approximately linear for tea. [3]
The other found that cognition improved with intake of cocoa flavanols up to quite high doses – elderly given 1 g/day cocoa flavanols performed significantly better on cognitive tests than those given lower doses. [4]
Unfortunately I don’t know what fraction of chocolate is made of flavanols. I’m guessing it’s not more than a few percent, in which case this research suggests the optimal dose of chocolate may be 50 g/day or more.
Chocolate in Support of Circadian Rhythms
Most authors attribute the benefits of chocolate to their flavanols, which are thought to improve endothelial function and increase blood flow to the brain, among other effects.
However, there are other active compounds in chocolate, include peptides that interact with the opioid receptor. The opioid receptor has a role in circadian rhythms, which is one reason low-dose naltrexone (which blocks opioid function at night) works. It’s possible that eating chocolate during the day may support circadian rhythms via opioid receptor stimulation, especially if the peptides can reach the systemic circulation.
Indirect evidence that this may be beneficial comes from a Russian study in which exorphins (opioid receptor ligands) were injected into rats:
The chronic intraperitoneal administration of the peptide at the same dose of 5 mg/kg significantly increased exploratory activity, decreased anxiety, and improved learning. [5]
I don’t know how much chocolate would have to be eaten to achieve a similar exorphin dose in humans, but I imagine it’s large.
Chocolate in Support of Nobel Prizes
So how shall we resolve the issue of optimal chocolate dose? For me, the decisive evidence comes from a recent study by Franz Messerli published in the New England Journal of Medicine.
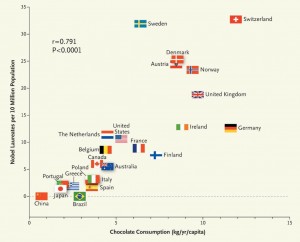
Based on chocolate’s support for cognitive function, he decided to see if chocolate consumption was related to another measure of cognition – Nobel Prize awards per capita. He counted Nobel Prizes and compared them to the recipient’s country’s chocolate consumption. These were his findings [6]:
There is clearly a strong correlation. The correlation coefficient is .79; p < 0.0001.
The correlation coefficient if Sweden is removed increases to .86 – which is suspicious:
Given its per capita chocolate consumption of 6.4 kg per year, we would predict that Sweden should have produced a total of about 14 Nobel laureates, yet we observe 32. Considering that in this instance the observed number exceeds the expected number by a factor of more than 2, one cannot quite escape the notion that either the Nobel Committee in Stockholm has some inherent patriotic bias when assessing the candidates for these awards or, perhaps, that the Swedes are particularly sensitive to chocolate, and even minuscule amounts greatly enhance their cognition. [6]
Those dastardly Swedes! Giving themselves more Nobel Prizes than their chocolate consumption warrants!
But I apologize, I’ve been diverted. The key point is, is there an optimum chocolate consumption?
the dose–response curve reveals no apparent ceiling on the number of Nobel laureates at the highest chocolate-dose level of 11 kg per year. [6]
11 kg/yr is an average of 30 g/day. So benefits are still increasing at that dose.
Of course, this was only a population level study. We still need to measure the doses in individual laureates to gain confidence. But anecdotally, there appears to be a correlation:
“I attribute essentially all my success to the very large amount of chocolate that I consume,” said Eric Cornell, an American physicist who received the Nobel Prize in 2001. “Personally I feel that milk chocolate makes you stupid. Now dark chocolate is the way to go. It’s one thing if you want like a medicine or chemistry Nobel Prize…but if you want a physics Nobel Prize it pretty much has got to be dark chocolate.”
Dark chocolate is, indeed, the PHD-approved form of this highly beneficial food.
Conclusion
This dose-response data might not be strong enough to define an RDA, but I’m going to take a stand: Bret’s intake of 35 g/day is healthy. Indeed, it’s right in line with the Nobel Prize-maximizing chocolate intake of the Swiss.
In regard to your last question, Bret – can you eat less chocolate, or none at all – the answer is clear. Yes, you can. But you must accept the consequences. You probably won’t be winning the next Nobel Prize for Physics.
References
[1] Buitrago-Lopez A et al. Chocolate consumption and cardiometabolic disorders: systematic review and meta-analysis. BMJ. 2011 Aug 26;343:d4488. doi: 10.1136/bmj.d4488. http://pmid.us/21875885.
[2] Oba S et al. Consumption of coffee, green tea, oolong tea, black tea, chocolate snacks and the caffeine content in relation to risk of diabetes in Japanese men and women. Br J Nutr. 2010 Feb;103(3):453-9. http://pmid.us/19818197.
[3] Nurk E et al. Intake of flavonoid-rich wine, tea, and chocolate by elderly men and women is associated with better cognitive test performance. J Nutr. 2009 Jan;139(1):120-7. http://pmid.us/19056649.
[4] Desideri G et al. Benefits in cognitive function, blood pressure, and insulin resistance through cocoa flavanol consumption in elderly subjects with mild cognitive impairment: the Cocoa, Cognition, and Aging (CoCoA) study. Hypertension. 2012 Sep;60(3):794-801. http://pmid.us/22892813.
[5] Belyaeva YA et al. Effects of acute and chronic administration of exorphin C on behavior and learning in white rat pups. Moscow University Biological Sciences Bulletin Volume 64, Number 2 (2009), 66-70, DOI: 10.3103/S0096392509020035. http://www.springerlink.com/content/qt537481061656gt/?MUD=MP.
[6] Messerli FH. Chocolate consumption, cognitive function, and Nobel laureates. N Engl J Med. 2012 Oct 18;367(16):1562-4. doi: 10.1056/NEJMon1211064. Epub 2012 Oct 10. http://pmid.us/23050509.











Recent Comments