After two weeks of science-heavy posts, I thought I’d do something light: a picture show.
Paleolithic Body Shapes
It’s often said that Paleolithic men were large-boned and very muscular. For instance:
The limb bones of the early Upper Palaeolithic Gravettian people are not only large but also have massive muscle attachments.
This is true of some Paleolithic skeletons, including Neanderthals and Eastern Gravettians, but it is far from generally true. (Gravettian is the name for a European toolkit used between about 28,000 and 22,000 BC.)
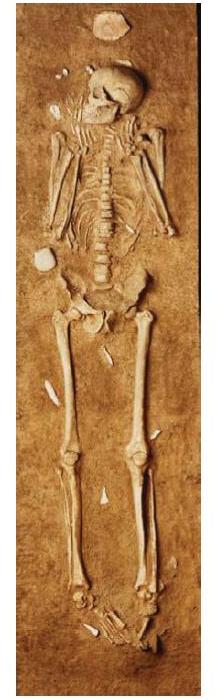
In fact, the Paleolithic population that expanded through Europe in the Upper Paleolithic was notably tall and slender. Here is a Gravettian skeleton from Grottes des Enfants 4 in Grimaldi, Italy [1]:
Note the narrow hips, narrow rib cage, and slender bones. The body shape is not dissimilar to some tall, slender East African populations today.
Of course, you can be small-boned and slender and strong. Still, it’s likely this population fought with poison-tipped throwing weapons like the atlatl, not with spears like the Neanderthals, and consequently needed much less strength. Also, they were fishermen and horticulturists, not big game hunters; their nitrogen isotope ratios prove that much of their protein came from fish.
What about obesity? Don Matesz had an interesting post this week, in which he argued that the Venus of Willendorf proves that Paleolithic Europeans were familiar with the shape of obese women:
Source: Wikipedia.
Indeed, it appears that post-menopausal weight gain may have been a problem on Paleolithic diets. As were sagging breasts:
Source: Wikipedia.
Paleolithic Art
The Gravettians were major producers of cave art. A few years ago, Friedrich Blowhard of 2blowhards.com did a great review of a book by anthropologist J. David Lewis-Williams, The Mind in the Cave: Consciousness and the Origins of Art, which sought to explain the origins of Paleolithic cave art.
Lewis-William’s idea is that is that the art was the product of shamanistic religious rituals involving hallucinations. The art was placed in the darkest and most inaccessible corners of caves because those were the best places to have a private hallucination.
Here is the Mammoth from Rouffignac:
To be sure, some of the cave art was in more accessible locations. Here is the Hall of the Bulls at Lascaux:
One thing I learned from Friedrich’s review is that animals were often painted as if they were floating in air – either lacking hooves, or with the bottoms of the hooves visible. Here is a bison at Altamira:
If Paleolithic art was created in an hallucinogenic state, it might explain The Sorcerer from Les Trois Freres:
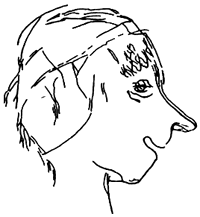
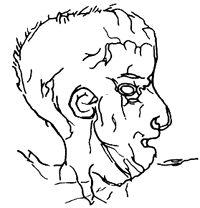
Paintings of humans were rare until the latest stages of the Upper Paleolithic. Via DonsMaps.com, here are some Paleolithic Frenchmen from La Marche, Vienne, France about 12,000 BC:
Paleolithic artists did sometimes represent the fish that made up so much of their diet. Here is a Paleolithic salmon:
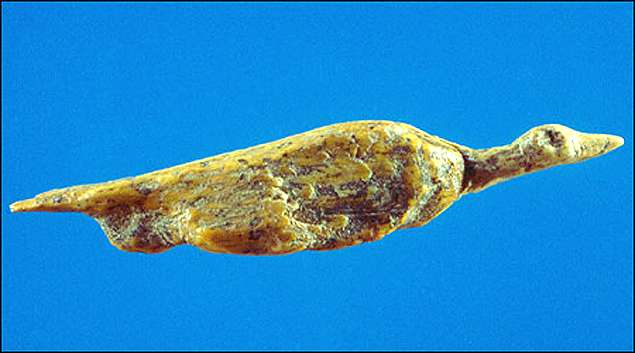
Here is a sculpture of a bird:
Conclusion
I’m happy to appropriate their diet, and I admire their art, but I must say, I’m quite pleased to be living now rather than then.
And now — that duck is making me hungry. It must be time for dinner!
References
[1] Holt & Formicola (2008) “Hunters of the Ice Age: The Biology of Upper Paleolithic People,” Yearbook of Physical Anthropology 51:70–99.





























Recent Comments