Here are items that caught my eye this week:
[1] Iodine watch! Japan fallout tracker: So you got potassium iodide somehow, and want to know whether to take it. Here’s an animated gif from the Central Institute for Meteorology and Geodynamics (ZAMG) in Austria that shows the radiation plume from the Japanese reactors. Luckily, the prevailing westerly winds have to date been blowing the radioactivity out to sea. Unluckily, the forecast is for winds to calm this weekend, which may direct the plume toward Tokyo by Sunday.
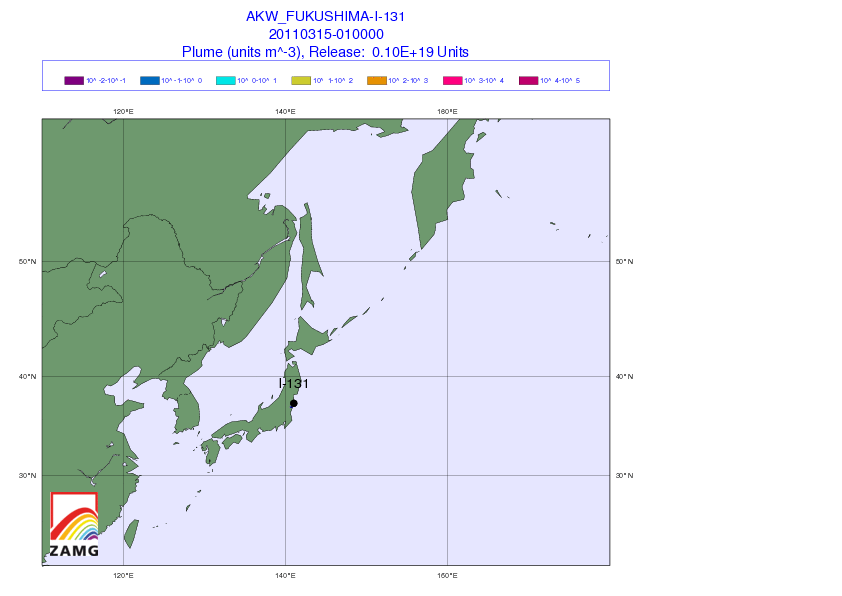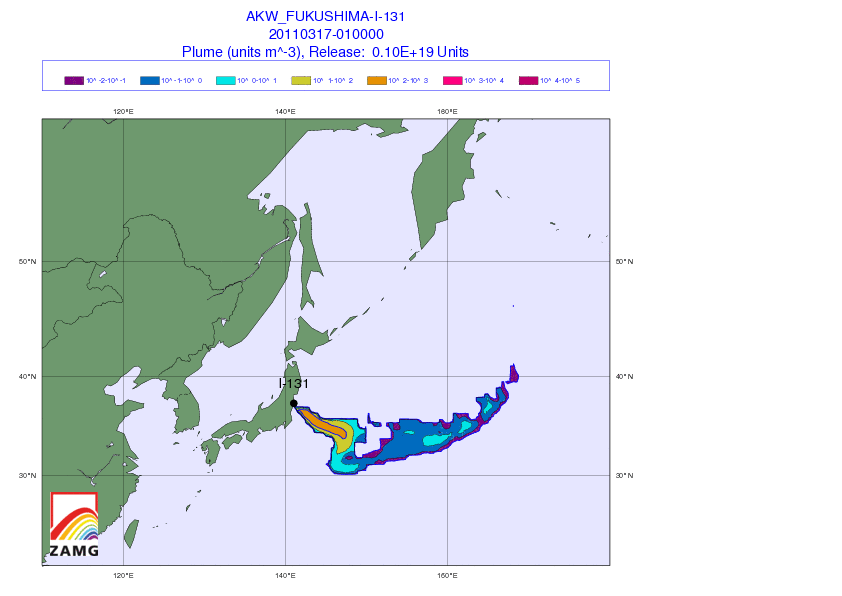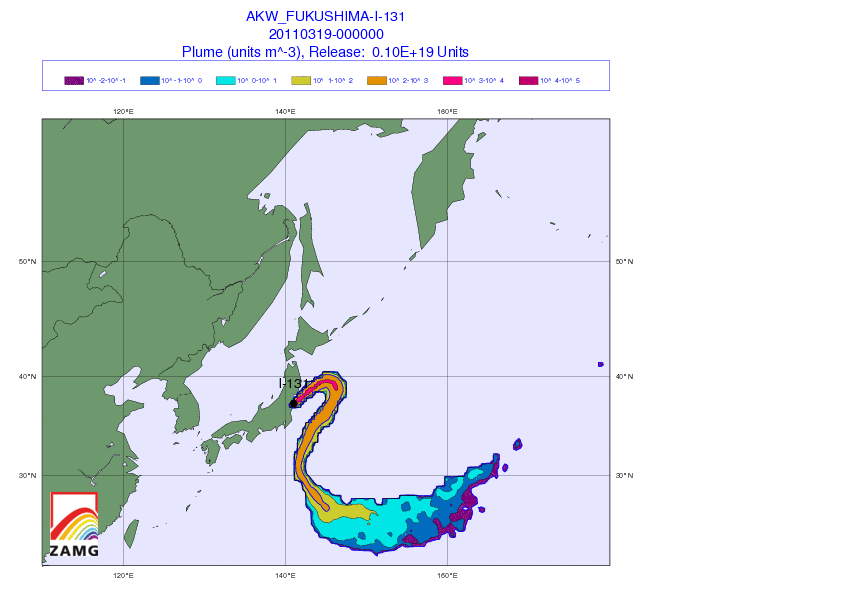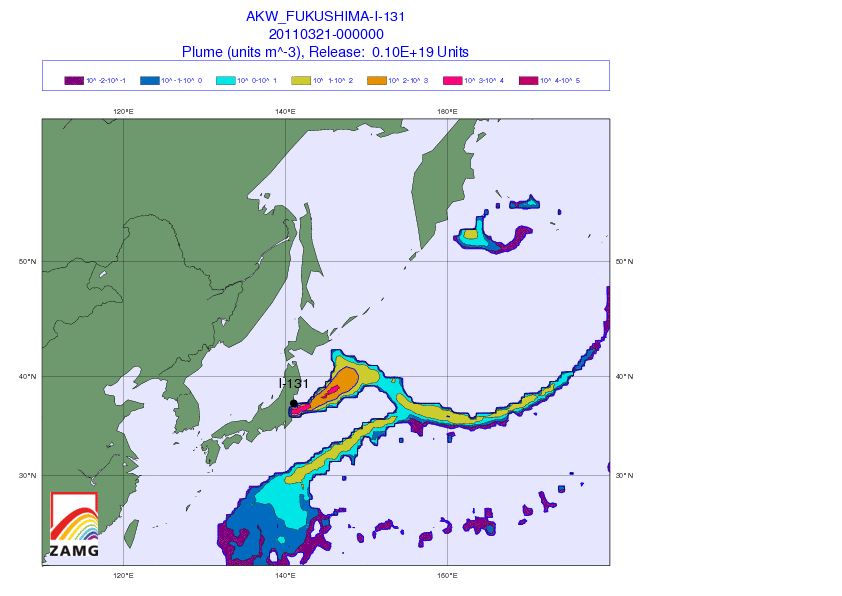
(via Zero Hedge)
The danger is not negligible for the Japanese. Over 4,200 tons of radioactive material are present at the Fukushima site – 24 times the amount present at Chernobyl – although in general the material is less radioactive (due to having already decayed significantly) than the Chernobyl materials. I hope that the world’s potassium iodide supplies are being directed to Japan at the moment. It would be a shame for them to be short-handed.
[2] Panic! Salt shortage in China: The Chinese may be over-reacting to the reactor story. Here they are mobbing a salt vendor in search of iodized salt:

A technician in my wife’s lab reports that her mother-in-law in China bought 20 bags of iodized salt – a lifetime supply – last week, just to be “safe.”
Let’s hope no one dies of salt toxicity trying to protect themselves from radioactivity!
Panic buying is not confined to China. Americans are paying exorbitant prices for iodine, even though the radiation danger here is almost non-existent. The price of the Iodoral tablets we recommend has tripled on Amazon; FDA-approved iodine supplements have risen in price almost 20-fold.
[3] Animal photo: Bad news calls for a hug:
[4] Used copies for sale?: If anyone wants to sell their copy of our book, Zoë would like to hear from you!
[5] Mmmmmmm!: If Sunday is too far away and you need a food post, Guy Giard has your fix. To work up an appetite, click on the cute couple:
[6] How was your meat glue?: As if we didn’t already have enough reasons to cook at home, here’s a new one. Restaurants not only use bad oils and MSG, some of them save money by buying recycled meat scraps, re-assembled into a facsimile of fresh meat through the use of “meat glue” – enzymatic treatment with tissue transglutaminase.
Tissue transglutaminase will be familiar to readers of our book as a primary player in gluten autoimmunity. It is expressed whenever wounds need repair, and helps cross-link proteins. This allows it to knit meat pieces together so they appear like natural flesh.
The trouble is that bacteria collect on the surface of meat. With a whole piece of meat, it is normally sufficient to cook the surface; rare meat is safe, since cooking kills the surface bacteria and the uncooked interior was antiseptic.
But when many small scraps are knitted together this way, the bacteria are retained in the interior of the meat. If the whole “steak” is not thoroughly cooked, bacteria will not be killed and the meat will be infected and unsafe.
Here is a video from Australian TV. Can you tell the real meat from the glued scraps?
[7] New foods to try: Melissa McEwen recommends fermented rice foods: “Indian Idli, which Stephan has blogged about … [is] SO DELICIOUS…. Filipino Puto [is] SO chewy and delicious with butter!… There is also some evidence that fermented rice improves cholesterol markers and reduces fatigue in animals.”
[8] Brain-Gut connections: It seems that trauma to the brain induces a leaky gut within 6 hours. I would never have guessed this as a cause of leaky gut. (Via Chris Kresser)
[9] Good news for Short People: Being small might be an advantage.
[10] True: “No lesson seems to be so deeply inculcated by experience of life as that you should never trust experts. If you believe doctors, nothing is wholesome; if you believe theologians, nothing is innocent; if you believe soldiers, nothing is safe.” – Lord Salisbury
[11] Top posts: Chris Masterjohn has a superb post, “Genes, LDL-Cholesterol Levels, and the Central Role of LDL Receptor Activity In Heart Disease”. It is too rich to summarize, but the best post I read this week. Also, Chris Kresser is nearing the end of his “9 Steps to Perfect Health” series (I’m jealous! We only had four steps.) This week he advises “Get More Sleep”.
[12] Almost the Top Post: Maybe I should buy some crickets. It seems hunting crickets is a very effective way to relieve stress – at least for cats. Mark Sisson’s friend’s cat recovered from disease by hunting crickets. What do you think? Will it work for people too?
[13] Lower Manhattan from Brooklyn Bridge Park:
(via Craig Newmark)
[14] Not the weekly video: Are earthquakes predicted by high tides, fish kills, whale beachings, homing pigeons going astray, and clockwise rotation of earthquakes around linked faults?
If so, there might be shaking on the west coast of North America this week:
[15] Weekly video: After all this disaster talk we can use a little fun. Here’s Dean Martin and Goldie Hawn, flirtatious and funny, from Rowan & Martin’s Laugh-In:





























Recent Comments