On Tuesday (Omega-3 Fats, Angiogenesis, and Cancer: Part I, April 26, 2011) I introduced the issue of possible relationships between omega-3 fatty acids, their lipid peroxidation products, and diseases of angiogenesis such as cancer, and promised to discuss a possible mechanism today.
It may be well, however, to start by saying a little bit more about the Brasky paper [1] linking prostate cancer to DHA.
Denise Minger’s Commentary on the Brasky Paper
Denise Minger wrote a commentary on this paper for Mark’s Daily Apple, which is excellent. Her conclusion – “given the oxidation-prone nature of all polyunsaturated fats, a massive intake of omega-3’s – despite their brilliance in moderation – could potentially do more harm than good” – is the proper one.
A few of Denise’s observations, however, could stand elaboration.
The study measured the fraction of serum phospholipid fatty acids in various polyunsaturated and trans-fat species, not dietary intake. This is the right parameter to measure, as fatty acid profiles can be measured precisely while dietary intakes assessed through questionnaires are notoriously unreliable. Also, phospholipids are the fats in cell membranes, and these are the ones involved in the inflammatory signaling pathways long thought to drive cancer risk. So cell membrane lipid measurements have the best chance to demonstrate a link to cancer risk.
Denise makes the important point, however, that the connection between dietary fish oil intake and serum fatty acid profile is not simple. Higher DHA intake raises phospholipid DHA levels, but lower intake of non-omega-3 fats also raises the DHA fraction. She points to a study [2] comparing a low-fat diet (20% fat, 6.7% PUFA, n-6:n-3 ratio 11.1) to a high-fat diet (45% fat, 15% PUFA, n-6:n-3 ratio 12.3). The low-fat diet had more of its fat in the form of long-chain omega-3s, but the specific DHA intake on the diets was not reported. Membrane DHA ended up 28% higher on the low-fat diet.
So if DHA is dangerous, low-fat dieters will be in the most trouble. Another reason to eat a high-fat diet!
Does this affect our interpretation of the Brasky study? I don’t think it affects it much, because study participants were healthy at the start of the study with no history of cancer and macronutrient intakes don’t vary a lot among the general public. Americans vary surprisingly little from the median of about 50% carbs, 15% protein, and 35% fat – so it’s likely that the quartile with high tissue DHA levels were also high fish oil consumers.
However, study participants were followed for 7 years, at which point their prostate cancer status was assessed. Incidence of low-grade prostate cancers had no association with start-of-the-study DHA intake, but incidence of high-grade prostate cancers was strongly associated.
Here are a couple of possible explanations for this pattern:
- DHA is bad: DHA doesn’t drive early cancer development but does drive late-stage cancer growth – i.e. the transition from low-grade to high-grade cancer. So the DHA consumers got the high-grade cancers. Angiogenesis does, in fact, drive the shift from low-grade to high-grade cancer, so a DHA-angiogenesis association would be consistent with this explanation.
- Hospital diet advice is bad: DHA was a marker at the start of the study for conscientious, educated, disciplined persons who followed health advice and ate fish oil. When these people were diagnosed with low-grade cancer, they followed the dietary advice of their cancer dietitian. The dietitian’s advice? Eat lots of wheat, whole grains, legumes, and vegetable oils. It could be the conscientious folks who followed bad diet advice from the hospital dietitian who got the high-grade cancers.
So there is a possible confounding effect.
Another of Denise’s assertions is that there is an “otherwise consistent train of research showing that DHA seems protective at best (and neutral at worst).” Now it is true that there are more studies showing DHA to have benefits against cancer than harm. But this trend is hardly consistent, and the vast majority of studies have failed to detect a relationship.
In the comments to Tuesday’s post, eric linked to a 2005 meta-review of studies on omega-3 fats and cancer. [3] The reviewers looked at 1,210 journal articles and found a mixed bag of mostly insignificant evidence:
Significant associations between omega-3 consumption and cancer risk were reported for lung cancer in two studies; for breast cancer in one; for prostate cancer in one; and for skin cancer in one. However, for lung cancer, one of the significant associations was for increased cancer risk and the other was for decreased risk (four other risk ratios were not significant for lung cancer). For breast cancer, five other estimates did not show a significant association. Only one study assessed skin cancer risk. No effects were reported for cancers of the aerodigestive tract, bladder cancer, colorectal cancer, lymphoma, ovarian cancer, pancreatic cancer, or stomach cancer. Thus, omega-3 fatty acids do not appear to decrease overall cancer risk.
Data were insufficient to permit assessment of a temporal or dose-response relationship. [3]
So the score was 4 studies finding that DHA is associated with less cancer, 1 that it is associated with more, and a boatload that it had no association.
Now there are two ways of interpreting this general insignificance of DHA against cancer. One is to note that there are slightly more studies showing DHA to have benefits than harm, and therefore to judge that DHA might be helpful against cancer.
But another, equally plausible, interpretation is this. Most Americans eat far too much omega-6, and their omega-6 to omega-3 tissue ratio is too high, which is pro-inflammatory via the COX-2 pathway. Eating omega-3s including DHA reduces inflammation by downregulating the COX-2 pathway. This accounts for the well-attested benefits of DHA against cardiovascular disease. Now, cancer is promoted by COX-2 pathway inflammation, which is why COX-2 inhibitors such as aspirin and ibuprofen are protective against cancer. [4] DHA’s action to downregulate this pathway must generate an anti-cancer effect. But, unlike aspirin and ibuprofen, DHA has no observable effect on overall cancer risk. This suggests that DHA has other effects, unrelated to its anti-inflammatory activity, that are cancer promoting. These counterbalance the benefits from its anti-inflammatory effect. If DHA has pro-angiogenic effects that are independent of COX-2 mediated inflammation, then this could account for the observations.
One reason an association of DHA with high-grade cancer may have been missed is that it would be detected only in large studies able to segregate cancers by grade. Brasky et al note:
In the European Prospective Investigation into Cancer and Nutrition (EPIC) (12), the highest quintile of percent DHA was associated with elevated risks of both low-grade (relative risk (RR) = 1.53, 95% CI: 0.96, 2.44) and high-grade (RR = 1.41, 95% CI: 0.76, 2.62) prostate cancer. They also reported significant positive associations of the percent EPA with high-grade prostate cancer (RR = 2.00, 95% CI: 1.07, 3.76). Given that the Prostate Cancer Prevention Trial and the European Prospective Investigation into Cancer and Nutrition, the 2 largest studies of blood levels of phospholipid fatty acids, reported increased risks of high-grade prostate cancer with high levels of ω-3 fatty acids, it remains a possibility that these fatty acids promote tumorigenesis. [1]
If there were no other evidence linking DHA to angiogenesis, the Brasky and EPIC study associations would be interesting, but unlikely to change anyone’s mind. Denise points out the need for other evidence – especially, mechanistic evidence – to make the connection more plausible:
We haven’t sleuthed out any mechanism that could explain why DHA (but not other polyunsaturated fats) promotes rapid tumor growth.
And this is where today’s post comes in. In fact, there is a known mechanism by which DHA but not other polyunsaturated fats can promote rapid tumor growth. Shou-Ching told me about it a few months ago.
DHA and Angiogenesis in Macular Degeneration
Let’s start by going back to 2003 and a paper on the role of a compound called carboxyethylpyrrole (CEP) in age-related macular degeneration (AMD). [5] AMD is an eye disease caused by improper angiogenesis. Basically, malformed blood vessels overgrow the eye, causing retinal detachment and blindness. It afflicts 35% of those over age 75, and is the leading cause of blindness in developed countries. CEP? Well, the paper explains:
Free radical-induced oxidation of docosahexaenoate (DHA)-containing lipids generates ω-(2-carboxyethyl)pyrrole (CEP) protein adducts that are more abundant in ocular tissues from AMD than normal human donors…. The CEP adduct uniquely indicates oxidative modification from DHA derivatives because CEP protein modifications cannot arise from any other common polyunsaturated fatty acid. [5]
CEP is uniquely produced by oxidation of DHA, not other PUFAs. Its abundance depends on DHA abundance, availability of retinyl proteins, and the level of oxidative stress.
CEP is elevated in AMD. The correlation is strong: a person in whom the immune system is trying but failing to clear elevated CEP levels almost invariably has macular degeneration (AMD):
Of individuals (n = 13) exhibiting both antigen and autoantibody levels above the mean for non-AMD controls, 92% had AMD. [5]
So CEP is a great marker for AMD. Is it causal?
Well, first it’s worth noting that the retina is uniquely vulnerable to DHA oxidation:
Although rare in most human tissues, DHA is present in ~80 mol % of the polyunsaturated lipids in photoreceptor outer segments (13). The abundance of DHA in photoreceptors, the high photooxidative stress in retina, and the fact that DHA is the most oxidizable fatty acid in humans (13) suggests that DHA oxidation products may have possible utility as biomarkers for AMD susceptibility. [5]
Oxidation is linked to AMD, and antioxidants slow AMD progression:
Oxidative damage appears to contribute to the pathogenesis of AMD (4) based on epidemiological studies showing that smoking significantly increases the risk of AMD (1, 24). The molecular mechanism for how smoking enhances the risk for AMD is not known. We speculate that reactive oxygen and nitrogen species derived from tobacco smoke in the lungs leads to oxidative protein modifications in the blood that contribute to drusen formation and choroidal neovascularization. Results from a recent clinical trial (5) also demonstrate that the progression of AMD can be slowed in some individuals by high daily doses of antioxidant vitamins and zinc. Direct evidence of oxidative damage in AMD donor eye tissues include elevated levels of CEP adducts uniquely derived from the oxidative fragmentation of DHA (6). [5]
This is where things stood in 2003. By 2010 this group, led by Case Western Reserve University chemist Robert G. Salomon, had established that administering CEP to mice can cause AMD:
To test the hypothesis that this hapten is causally involved in initiating an inflammatory response in AMD, we immunized C57BL/6J mice with mouse serum albumin (MSA) adducted with CEP. Immunized mice develop antibodies to CEP, fix complement component-3 in Bruch’s membrane, accumulate drusen below the retinal pigment epithelium during aging, show decreased a- and b-wave amplitudes in response to light, and develop lesions in the retinal pigment epithelium mimicking geographic atrophy, the blinding end-stage condition characteristic of the dry form of AMD. Inflammatory cells are present in the region of lesions and may be actively involved in the pathology observed. [6]
This constitutes the first really good animal model for AMD. [6]
How does this relate to cancer? That leads us to a Nature paper from October 2010 [7], from the group of Tatiana Byzova at the Cleveland Clinic.
DHA, Immunity, and Angiogenesis
This is a rich paper. Briefly, CEP has a physiological function: it is transiently elevated in wounds and recruits immune cells from bone marrow to the site of the wound. These immune cells further increase oxidative stress and promote angiogenesis; CEP levels are highest at the time of peak angiogenesis. CEP is highly elevated in cancers. Unlike in wounds, where CEP is elevated for a few days, in cancers CEP elevation is chronic.
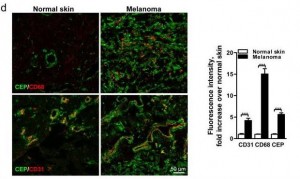
Here’s a staining comparing CEP in normal skin and in melanoma:
The CEP is co-localized with CD68, a glycoprotein which binds to LDL and is found on macrophages, and with CD31, a membrane marker of neutrophils, macrophages, and endothelial cells. CEP is marking endothelial cells and white blood cells in angiogenic vessels, and possibly LDL.
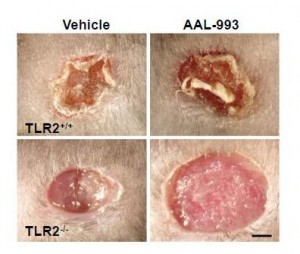
It turns out that CEP drives angiogenesis by attaching to an immune receptor, Toll-like receptor 2 (TLR2). There are two major pathways for angiogenesis: one driven by vascular endothelial growth factor (VEGF), which is dominant in conditions of hypoxia (oxygen starvation), and one by TLR2. Of these, the TLR2 pathway may in some contexts be more important. Here are pictures of wound healing in mice:
On the upper left is a normal mouse. On the upper right is a similar wound treated with the VEGF inhibitor AAL-993. This wound is rather like a cancer treated with the VEGF inhibitor Avastin. Wound healing is slightly impaired, but still works.
On the lower left is a similar wound with no VEGF inhibition, but the TLR2 pathway blocked by TLR2 knockout. The wound can’t scab and doesn’t heal successfully. If TLR2 is knocked out and VEGF inhibited, there is no wound healing at all (lower right).
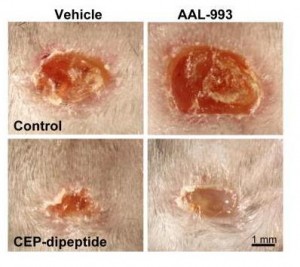
You can accelerate angiogenesis and wound healing by adding CEP to the wound.
In the bottom row, CEP has been added. Left is without VEGF inhibition, right with.
If you administer CEP-neutralizing antibodies to a normal wound, wound healing takes more than twice as long. This confirms that angiogenesis driven specifically by CEP (and therefore by DHA oxidation) is part of healthy wound healing.
Tumors use these same pathways to generate vessels and feed their growth. As the paper notes:
[T]umors implanted in TLR2-/- mice exhibited dramatically decreased vascularization and increased areas of necrosis. [7]
Here’s the paper’s conclusion:
Altogether our results establish a novel mechanism of angiogenesis that is independent of hypoxia-triggered VEGF expression. The products of lipid oxidation are generated as a consequence of oxidative stress and are recognized by TLR2, possibly in a complex with TLR1 on ECs, and promote angiogenesis in vivo, thereby contributing to accelerated wound healing and tissue recovery. If high levels of CEP and its analogs accumulate in tissues, it may lead to excessive vascularization, e.g. in tumors. Contribution of the CEP/TLR2 axis to angiogenesis varies in different physiological settings possibly depending on the extent of oxidative stress. CEP-driven angiogenesis may be an attractive therapeutic target, especially in cancers resistant to anti-VEGF therapy. Inflammation and oxidation-driven angiogenesis may occur in other pathologies, for example atherosclerosis, where arterial thickening can depend on its microvasculature. In these settings, there is an extensive generation of oxidative products which might promote atherogenesis via TLR2. Indeed, it was shown that TLR2?/? mice are protected from atherosclerosis, and this effect could be mediated by cells other than bone marrow-derived29. Thus, along with pathogen- and danger-associated molecular patterns, TLR2 recognizes an oxidation-associated molecular pattern. This new function of TLR2 as a sensor of oxidative stress reveals the shortcut link between innate immunity, oxidation and angiogenesis. [7]
Connection to Vitamin A
DHA is oxidized to a compound called HOHA which then combines with a protein, generally a retinyl (vitamin A-derived) protein to form CEP.
Cancers generate lots of CEP from DHA, and perhaps one way they do that is by generating lots of retinyl proteins. Cancers are known to have disturbed vitamin A biology with lots of retinyl:
Disturbance in vitamin A metabolism seems to be an important attribute of cancer cells. Retinoids, particularly retinoic acid, have critical regulatory functions and appear to modulate tumor development and progression. The key step of vitamin A metabolism is the esterification of all-trans retinol, catalyzed by lecithin/retinol acyltransferase. In this work we show that malignant melanoma cells are able to esterify all-trans retinol and subsequently isomerise all-trans retinyl esters into 11-cis retinol, whereas their benign counterparts – melanocytes are not able to catalyze these reactions. Besides, melanoma cell lines express lecithin/retinol acyltranseferase both at the mRNA and protein levels. In contrast, melanocytes do not express this enzyme … [8]
I haven’t looked much into this literature but it may speak to higher cancer risk with excessive vitamin A intake. Thus high-vitamin A cod liver oil may be a double risk for cancer patients.
Conclusion
It looks like we have a recipe for angiogenesis:
This recipe is invoked normally and properly during wound healing. But it is also invoked excessively in pathological contexts – notably in cancers and age-related macular degeneration, probably also in other angiogenesis-associated diseases such as arthritis, rosacea, obesity, psoriasis, endometriosis, dementia, and multiple sclerosis.
In the case of cancer, DHA oxidation to CEP might transform miniscule, harmless cancers to high-grade, life-threatening cancers.
Should this possibility affect our dietary omega-3 recommendations? Well, we need to know the relative importance of the three ingredients on the left side of the above equation in producing angiogenesis. Chris Kresser wondered in the comments Tuesday whether oxidation may be the key factor:
I question whether DHA supplementation would truly play a causative role in the absence of a *pro-oxidative environment*.
In other words, perhaps in someone eating a SAD, not exercising, under a lot of stress, etc. DHA is more easily oxidized and thus potentially carcinogenic.
But in someone that is keeping all other oxidative risk factors low (i.e. they’re avoiding n-6, exercising, managing stress, reducing exposure to chemical toxins, etc.) I tend to doubt that supplementing with DHA could cause significant harm.
That’s the last piece of the puzzle: how do we minimize the level of oxidized DHA?
As I replied to Chris in the comments, low-carb Paleo dieters are not out of the woods in regard to oxidative stress. Oxidative stress is generated normally during metabolism, immune function – and by cancers. If anti-oxidant minerals like zinc, copper, and selenium and vitamins like vitamin C are deficient, then oxidative stress can be very high on a low-carb Paleo diet.
At the moment, I think it’s prudent to eat no more than 1 pound of salmon or similar cold-water fish per week, to avoid further EPA/DHA supplements, and to avoid low-fat diets which tend to elevate membrane DHA levels. Moderate omega-3 consumption is especially important for those suffering from diseases of pathological angiogenesis – especially cancer. DHA is essential for good health – but in excess, it is probably dangerous.
References
[1] Brasky TM et al. Serum Phospholipid Fatty Acids and Prostate Cancer Risk: Results From the Prostate Cancer Prevention Trial. Am. J. Epidemiol. April 24, 2011 DOI: 10.1093/aje/kwr027 (Will be at http://pmid.us/21518693.)
[2] Raatz SK et al. Total fat intake modifies plasma fatty acid composition in humans. J Nutr. 2001 Feb;131(2):231-4. http://pmid.us/11160538.
[3] MacLean CH, Newberry SJ, Mojica WA, et al. Effects of Omega-3 Fatty Acids on Cancer. Summary, Evidence Report/Technology Assessment: Number 113. AHRQ Publication Number 05-E010-1, February 2005. Agency for Healthcare Research and Quality, Rockville, MD. http://www.ahrq.gov/clinic/epcsums/o3cansum.htm.
[4] Harris RE. Cyclooxygenase-2 (cox-2) and the inflammogenesis of cancer. Subcell Biochem. 2007;42:93-126. http://pmid.us/17612047.
[5] Gu X et al. Carboxyethylpyrrole protein adducts and autoantibodies, biomarkers for age-related macular degeneration. J Biol Chem. 2003 Oct 24;278(43):42027-35. http://pmid.us/12923198.
[6] Hollyfield JG et al. A hapten generated from an oxidation fragment of docosahexaenoic acid is sufficient to initiate age-related macular degeneration. Mol Neurobiol. 2010 Jun;41(2-3):290-8. http://pmid.us/20221855.
[7] West XZ et al. Oxidative stress induces angiogenesis by activating TLR2 with novel endogenous ligands. Nature. 2010 Oct 21;467(7318):972-6. http://pmid.us/20927103.
[8] Amann PM et al. Vitamin A metabolism in benign and malignant melanocytic skin cells: Importance of lecithin/retinol acyltransferase and RPE65. J Cell Physiol. 2011 Apr 4. doi: 10.1002/jcp.22779. [Epub ahead of print] http://pmid.us/21465477.











Hi Paul,
I agree, we are encouraged to take too much of it. I heard that around 1000 mg of fish oil a day was ok.
I also came across this link “Effects of Omega-3 Fatty Acids on Cancer”:
http://www.ahrq.gov/clinic/epcsums/o3cansum.htm
Do you have any views on ALAs regarding safety and cancer risk?
I just remembered you mentioned that last link in your post.
Hi Jeff,
I think ALA is usually beneficial in small to moderate amounts but I don’t go out of my way to get it. It seems to be good to have LA and ALA in some sort of balance.
Interesting. On the issue of Vitamin A; retinol is toxic in combination with high alcohol intake, a little-known fact which has tended to push down the RDA to deficiency levels in recent years.
Retinoic acid helps TGF-beta induced activation of Treg (T regulatory) cells, which reduces inflammation, improves Th1 immunity and helps account for the anticancer effects of retinol.
Cod liver oil use by alcoholics seems a bad idea, but not by cancer patients;
Int J Cancer. 2009 Sep 1;125(5):1155-60.
Cod liver oil, other dietary supplements and survival among cancer patients with solid tumours.
Skeie G, Braaten T, Hjartåker A, Brustad M, Lund E.
SourceInstitute of Community Medicine, University of Tromsø, Norway. guri.skeie@uit.no http://www.ncbi.nlm.nih.gov/pubmed/19444919
Abstract
The effect of various dietary supplements on chronic diseases and mortality has been widely studied, but few convincing results have emerged from studies in well-nourished populations. In Norway, both cod liver oil and other dietary supplements are frequently used. In the Norwegian Women and Cancer cohort study, we explored if supplement use before diagnosis affected survival of cancer patients with solid tumours. We performed Cox proportional hazards analyses, adjusting for age at diagnosis, smoking and stage. Cod liver oil was the most frequently used dietary supplement, followed by multivitamins and minerals. Whole year daily use of cod liver oil was associated with lower risk of death in patients with solid tumours [RR = 0.77 (95% CI 0.61-0.97)] and in lung cancer patients [RR=0.56 (95% CI 0.34-0.92)]. Also daily and occasional use of other dietary supplements decreased the risk of death among lung cancer patients [RR = 0.70 (95% CI 0.49-0.99) and 0.55 (95% CI 0.31-0.97)]. More research is needed to clarify the association; meanwhile adjustment for dietary supplement use should be performed in survival analyses of lung cancer patients.
Thank you, George. Very interesting.
Hepatotoxicity of alcohol-induced polar retinol metabolites involves apoptosis via loss of mitochondrial membrane potential
http://www.fasebj.org/content/19/7/845.full
The aims of our study were to 1) establish whether the enhanced vitamin A hepatotoxicity in alcoholics could be related to toxic polar retinol metabolites (PRM) generated by alcohol-induced microsomal enzymes and 2) assess the underlying mechanism of cell death.
PRINCIPAL FINDINGS
1. Identification and purification of polar retinol metabolites
Chronic alcohol consumption interferes with vitamin A metabolism and may lead to a striking vitamin A depletion due to a competitive antagonism for the same hepatic enzymes alcohol dehydrogenase (ADH) and cytochrome P4502E1 (CYP2E1). Vitamin A supplementation may correct this deficiency, but chronic alcohol consumption enhances the intrinsic hepatotoxicity of vitamin A. We have recently demonstrated in rat that ROH and RA are biotransformed by induced CYP2E1 into the PRMs 18-OH-RA and 4-oxo-RA, while the selective inhibition of CYP2E1 by chlormethiazole prevents PRM production.
“Thus high-vitamin A cod liver oil may be a double risk for cancer patients.”
A toxicity is D deficiency and vice versa: http://www.westonaprice.org/fat-soluble-activators/vitamin-a-on-trial
D deficiency doubles probability of many cancers: http://orthomolecular.org/resources/omns/v04n11.shtml
So these downsides of omega-3 may explain the difficulty in the conversion of ALA into EPA and DHA.
Those whose only dietary fat come from flaxseed may be protected by this “defect” in human physiology more than by avoiding lean, animal food.
It’s really amazing how many checks and balances are built-in in the human body.
I think the Internet is giving proof that we all should be more respectful of our own bodies, especially Doctors who play politics.
DHA+Vitamin A = Eggs?