Mario’s post last Thursday (Low Carb High Fat Diets and the Thyroid, Aug 18, 2011), looking at a series of studies cited in a July 1 post by Anthony Colpo, elicited a reply from Anthony.
The exchange turned out to be a blessing, because it is generating some insights on topics of fundamental importance.
Low-Carb Dangers
A motivating factor for our book was Paul’s bad experience with very low carb dieting. We felt obliged to warn the Paleo community that it was possible to become deficient in glucose and that this could be dangerous. We’ve blogged about “Zero Carb Dangers” (see especially Dangers of Zero-Carb Diets, II: Mucus Deficiency and Gastrointestinal Cancers; Danger of Zero-Carb Diets III: Scurvy; Dangers of Zero-Carb Diets, IV: Kidney Stones). Our work has persuaded many in the Paleo and low-carb communities to eat more “safe starches” including white rice. We recommend a carb intake that approaches the body’s total glucose utilization.
We do recommend ketogenic diets and low-carb diets as therapies for many neurological disorders and some infections, but believe that even ketogenic diets should generally include at least 200 glucose calories per day.
So when we consider claims that low-carb diets can be dangerous, it’s not without sympathy.
At the same time, given the therapeutic potential of low-carb and ketogenic dieting, and the likelihood that humans are evolutionarily adapted to a range of macronutrient intakes, we don’t think it’s appropriate to repudiate low-carb entirely.
This places an onus on us to closely examine the evidence, understand precisely when a low-carb diet passes from healthy to unhealthy, and make clear recommendations for how much dietary glucose is needed in different circumstances to avoid negative consequences.
The Issue of Low-Carb and the Thyroid
One problem that sometimes occurs on low-carb diets is a syndrome called “euthyroid sick syndrome,” characterized by low T3 and high reverse T3 hormone levels and elevated cortisol.
Here’s our friend and gracious podcast host, Danny Roddy:
[A low-carb] diet will lead to an elevation of catabolic stress hormones, while [a high-carb diet] has been shown to increase thyroid hormone triiodothyronine (T3), increase testosterone, and decrease cortisol, the anti-hair, pro-misery stress hormone (here, here, here (PDF), & here).
And here’s Anthony Colpo in his July 1 post:
[D]ecreasing carbohydrate intake to low levels results in diminished levels of T3 and/or increased rT3, something most aspiring fat-burners wish to avoid desperately.
Few things are more essential to good health than proper thyroid function. So this is clearly an issue we have to investigate and understand. It could impact our prescription for the minimum level of carb intake needed for good health.
Why Mario’s Post Emphasized Omega-6 Fats
Anthony seemed to think that Mario’s emphasis on the dangers of high-omega-6 diets was intended as a denial that low-carb specifically could be the cause of thyroid trouble. No.
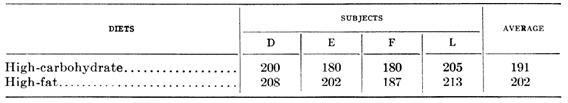
The studies in question compared low-carb high-fat diets with other diets. In some but not all studies, thyroid function was impaired on the low-carb high-fat diet.
In looking at the studies cited by Anthony plus a few others, Mario found that thyroid function was impaired on all of the low-carb high-omega-6 diets but none of the (admittedly few) low-carb high-saturated-fat diets.
This led him to emphasize the role of the fatty acid type, rather than the amount of carbohydrates. It is also supportive of Perfect Health Diet claims that high omega-6 is toxic whereas high saturated fat is not.
Mario did not have space to treat the dangers of glucose deficiency for the thyroid, especially since the studies he was examining did not provide compelling evidence about the effects of dietary carbohydrate restriction (once the possibility of PUFA toxicity was accounted for). So he didn’t venture into this question, other than to assert the importance of “moderate” carb consumption.
But now we will – in the remainder of this post, and two upcoming guest posts.
Let’s explore the circumstances under which we might expect low-carb diets to cause “euthyroid sick syndrome.”
The Limits to Glucose Production
Let me begin by revisiting the initial post in our Zero-Carb Dangers series (Dangers of Zero-Carb Diets, I: Can There Be a Carbohydrate Deficiency?, Nov 10, 2010). I’ve been meaning to correct that post for some time, and this seems a good occasion.
That post emphasized size of the liver as a limit on its ability to synthesize glucose from protein. There is, indeed, a physical limit to the liver’s ability to manufacture glucose from protein. As long as unlimited fat is available for energy production and unlimited protein is available as a gluconeogenic substrate, the limit is determined by the oxygen supply to the liver and is about 400 g / 1600 calories per day.
However, this theoretical limit is never reached in healthy humans, except in some diabetics (as Nigel Kinbrum pointed out). The liver’s conversion of protein to glucose is controlled hormonally; insulin and glucagon are the most important players, with insulin inhibiting gluconeogenesis and glucagon promoting it.
Diabetics can have very high rates of gluconeogenesis because their pancreatic beta cells may no longer produce insulin even as their pancreatic alpha cells continue to produce glucagon.
In normal healthy humans, basal hormone levels are balanced so that during fasting or starvation, the liver and kidneys manufacture minimal but sufficient glucose while sparing protein as much as possible.
In a normal person, the liver and kidneys together never produce more than about 100 g / 400 calories glucose per day from protein. In the absence of hormonal dysregulation, this is a fairly hard limit.
Glucose Utilization and Glucose Deficiency
We discussed this in the book, and I won’t repeat the evidence. Suffice it to say that everyone consumes at least 600 glucose calories per day, a majority by the brain but also extensive amounts for structural components of the body – glycosylated proteins which coat every cell, and glycoproteins which are major components of mucus, joint lubricants, and connective tissue – and for immune function (since glucose is needed to produce the reactive oxygen species that kill pathogens).
Because limited research has been done on this subject, it’s possible that we’ve underestimated the body’s glucose needs. It could be as high as 800 glucose calories per day. It’s not likely to be lower.
This is for sedentary healthy people. Two factors may substantially increase glucose utilization:
- Infection. Many pathogens consume glucose – indeed, people with parasitic infections can sometimes have great difficulty obtaining enough glucose from food – and the immune system also consumes glucose.
- Athletic activity. Exercise can consume large amounts of glucose.
Let’s look at athletic activity. The Mayo Clinic lists the amount of calories burned per hour of exercise of various kinds, and the most intense types of exercise, such as running or cycling at race speeds (>20 mph), may burn 1,200 calories per hour. Most of these calories come from fat, but 30-40% may come from glycogen, and glucose is consumed to replace the glycogen. Thus, a runner or cyclist may burn up to 400 glucose calories per hour of training.
In a cyclist, runner, or swimmer who trains 2 hours per day, therefore, glucose needs may be quite a bit higher than in our sedentary healthy person. Such an athlete may be consuming ~1500 glucose calories per day.
Yet at most 400 calories of glucose per day can be manufactured from protein. It’s clear that athletes need to eat a fair amount of carbohydrate to avoid a glucose deficiency.
Anthony Colpo is a cyclist and athlete who routinely engages in intense endurance exercise. His unusually high glucose utilization is presumably what made him vulnerable to glucose deficiency syndromes – and therefore more sensitive than others to the dangers of low-carb diets.
The Basis for Our Carbohydrate Consumption Recommendations
Let’s go back to our sedentary healthy person, and let’s consider the minimum dietary glucose that person needs to avoid difficulty.
First let’s consider a person who eats a large amount of protein – 600 calories per day, about double the intake of an average American.
Roughly 200 calories per day may be needed for structural uses, leaving 400 calories per day for possible conversion to glucose.
But if protein consumption is lower, there may not be enough substrate to create 400 glucose calories per day. This may lead to hormonal changes that try to conserve protein by limiting gluconeogenesis.
Now let’s look on the glucose side. If 600-800 glucose calories are utilized by the body daily, and at most 400 of those can be manufactured from protein and at most ~300 can be displaced by ketones, then someone on a zero-carb diet is living right on the margin of glucose deficiency.
And this is before athletic activity or infections are considered.
If 200 glucose calories per day are consumed, and if 400 protein calories are consumed, and if MCT oil (a ketogenic substrate) is consumed to make it easier to generate ketones to displace glucose, then one might just barely meet the body’s structural glucose and protein needs on a ketogenic diet. This is why we recommend that ketogenic diets include at least 200 calories from starch.
But it’s not really desirable to live on the margin of glucose deficiency, especially if you’re not making it easy for your body to generate ketones. For this reason, our normal diet recommends 400 calories or more from starches.
The Trouble with Vegetables
We generally advise not counting vegetables as carb calorie sources. This often puzzles diabetics who note that vegetables have some sugar – typically, about 80 calories per pound – and that consuming vegetables raises their blood sugar levels.
The reason we recommend not counting vegetable calories is that digestion of vegetable matter is an energy-intensive process that consumes glucose. Gut cells consume glucose directly, and also vegetables have a lot of fiber which causes gut bacterial activity which in turn leads to immune activity which consumes glucose. This glucose consumption by the gut and immune system occurs over an extended period of time after vegetables are eaten – perhaps 6 hours. But vegetable sugars are digested quickly – mainly in the first hour. So you can have a surge of blood sugar due to vegetable sugars, even if the vegetables make no net contribution to daily glucose balance.
So it’s really starches and fruits that are the useful sources for meeting the body’s carb needs.
What Happens When There is a Glucose Deficiency?
When the body is deficient in glucose, the hormonal milieu changes to help maintain body functions while conserving glucose and protein.
Two of the important hormones are cortisol and T3.
T3, the most active thyroid hormone, has a strong effect on glucose utilization. T3 stimulates glucose transport into cells, and transport is the limiting factor in glucose utilization in many cell types. In hyperthyroidism, a condition of too much T3, there are very high levels of glucose utilization. Administration of T3 causes elevated rates of glycolysis regardless of insulin levels.
The body can reduce T3 levels by converting T4 into an inactive form called reverse T3 (rT3) rather than active T3. High rT3 levels with low T3 levels lead to reduced glucose transport into cells and reduced glucose utilization throughout the body.
Cortisol is a hormone that helps prevent hypoglycemia, a condition of low blood glucose. It reduces glucose utilization and increases gluconeogenesis.
So the syndrome of low T3, high rT3, and high cortisol can be understood as a diagnostic pattern of a systemic glucose deficiency.
What Is “Euthyroid Sick Syndrome”?
Euthyroid sick syndrome is defined as “a state … where the levels of T3 and/or T4 are at unusual levels, but the thyroid gland does not appear to be dysfunctional.” Specifically, “Reverse T3 are generally increased signifying inhibition of normal Type 1 enzyme or reduced clearance of reverse T3. Generally the levels of Free T3 will be lowered.”
Wikipedia’s list of causes includes:
- Fasting, starvation (PAJ: These induce glucose deficiencies, especially if there is insufficient protein available to sustain even the normal 400 calories/day glucose synthesis.)
- Sepsis (PAJ: Infection increases glucose requirements.)
- Trauma (PAJ: Fabrication of structural glycoproteins and protein glycosylation is increased during wound repair.)
- Malignancy (PAJ: Cancers consume large amounts of glucose.)
- Hypothermia (PAJ: Shivering, like endurance exercise, consumes glycogen.)
- Cirrhosis (PAJ: Damage to the liver may reduce its ability to synthesize glucose, forcing glucose conservation.)
- Chronic renal failure (PAJ: The kidney is the other organ besides the liver that synthesizes glucose from protein. So kidney damage will reduce the body’s ability to synthesize glucose.)
Looking at this list, it seems that euthyroid sick syndrome may be just another name for a systemic glucose deficiency.
If glucose deficiency is the cause, then obviously low carb diets are going to be a risk factor for euthyroid sick syndrome.
This is not to say that low carb diets will automatically lead to euthyroid sick syndrome. A sedentary person free of infections may be quite normal and healthy on a very low carb diet. This is why most low carbers do not experience the condition.
But if other risk factors, like infection, cancer, or endurance exercise, are present, then the odds of developing euthyroid sick syndrome on a low carb diet may become quite high.
Diagnostic Value of rT3:T3 Ratio for Low-Carb Dieters
Here’s an interesting implication of today’s analysis.
The ratio of rT3 to T3 may have diagnostic value for glucose status and therefore for the presence of infections or cancers. It might not be a bad idea for low-carb dieters to monitor these hormone levels, and to eat enough “safe starches” to keep their rT3:T3 ratio at normal levels.
The rT3:T3 ratio is likely to be of much greater clinical value to low-carbers than to the average high-carb American. So even though doctors rarely test for it, low-carb dieters may find it quite useful.
Are High-Carb Diets Without Risk?
In the wake of Anthony’s reply I was amused by a Twitter conversation between @DannyRoddy and @StabbyRaccoon – two of the smartest and nicest people on the Web.
As noted above, Danny believes that high-carb diets might be beneficial by creating above-normal T3 levels:
I believe the real question is: what range radically increases T3?…
I’m more concerned where CHO starts dramatically increasing T3.
Stabby paid me the honor of valuing my opinion:
maybe Paul … could look into it.
Alright, let’s look (briefly) into it.
In the quote from Danny on potential risks of low-carb diets, he cited several papers. One of them was this:
To evaluate the effect of changes in dietary carbohydrate (CHO) and excessive caloric consumption on circulating thyroid hormone levels, six normal weight subjects were fed five separate diets: three isocaloric diets with 20%, 40%, or 80% CHO and two hypercaloric (+2000 calories) diets with 20% or 40% CHO for 5 days each as outpatients. T4, T3, and rT3 concentrations were measured in plasma samples collected on the morning of the sixth day. At least 1 week of the subjects’ usual diets intervened between each experimental diet.
Mean T4 and rT3 levels were similar after all diets. Pair-wise comparisons among all five diets revealed significantly (P < 0.005) increased T3 concentrations after both hypercaloric diets compared to the iso-20 and iso-40 diets, and after the iso-80 compared to the iso-20 diet. A multiple regression analysis of the data revealed the highest correlation of T3 levels with total calories (r = 0.68; P < 0.001) rather than with the intake of CHO (r = 0.46; P < 0.025), fat (r = 0.49; P < 0.02), or protein (r = 0.30; P = NS).
I haven’t read the full study yet and find this abstract mildly puzzling. On the one hand, the multiple regression analysis shows that fat, not carbohydrate, is the most effective macronutrient at raising T3. Maybe Danny should eat a high-fat diet to raise his T3.
On the other hand, the normo-caloric 80% carb diet had more T3 than the normo-caloric 20% carb diet. So maybe carbs do increase T3 more than fat.
Now, hypercaloric (positive energy balance) diets are associated with a variety of diseases including obesity and metabolic syndrome. Stephan Guyenet has argued that positive energy balance is itself inflammatory and damaging, and that high-reward foods which induce overeating may directly cause metabolic diseases.
In this study, T3 concentrations were increased similarly on both hypercaloric (2000 excess calories) 20% and 40% carb diets and normo-caloric 80% carb diets. Could it be that some of the ill effects of hypercaloric diets will also be present on normo-caloric high-carb diets?
Of course, with any hormone we have to ask what the right amount is. Usually both too much and too little are problematic. This is certainly true of thyroid hormones.
High T3 concentrations are characteristic of the disease hyperthyroidism and have negative effects. One of the effects of high T3 levels is enhanced transport of glucose into cells. For instance:
Pre-treatment of these cells with T3 moreover substantially enhances the stimulatory effect of insulin such that at maximally effective hormone concentrations the effects of T3 and insulin on glucose transport are more than additive and indeed nearly multiplicative …
The extra glucose transported into cells is disposed of through glycolysis. Glycolysis is the characteristic metabolism of cancer cells, so high T3 might promote the cancer phenotype.
Indeed, hyperthyroidism increases the risk of ovarian cancer by 80%.
Glycolysis also occurs in the cytosol, making glucose and downstream energy substrates like pyruvate and lactate available to bacteria. Thus, high T3 may promote bacterial infections.
Indeed, thyroid storms can cause sepsis.
Those who have been following CarbSane’s exposition of the dangers of lipotoxicity may be interested to find that high T3 not only increases circulating glucose levels and rates of glycolysis, but also circulating free fatty acid levels:
Hyperthyroidism, which was induced by administration of tri-iodothyronine (T3) to rats for 2, 5 or 10 days, increased fasting plasma concentrations of glucose, insulin and free fatty acids. Administration of T3 for 2 or 5 days increased the rates of glycolysis at all insulin concentrations studied …
Elevated free fatty acids seem to be the primary cause of diabetes. Here elevated free fatty acids are associated with high glucose and with a hormonal trait – high T3 – associated with high-carb diets.
(Aside: This kind of evidence is why we have to be a bit cautious in assuming that free fatty acid levels, and thus diabetes risk, are higher on low-carb high-fat diets. Recently CarbSane and I had a brief discussion on this topic: see this post on her blog and the comment thread. She leans toward the idea that more dietary fat = more free fatty acids and thus more lipotoxicity; to me the issue is far from clear, as the need to dispose of glucose will tend to inhibit drawdown of free fatty acids. I think that moderate carb consumption, near the body’s glucose utilization, rather than high carb consumption may minimize lipotoxicity. However, concerns over lipotoxicity might lead us to revisit our suggestion of ketogenic diets for diabetes.)
Getting back to the question Stabby asked me to look into: I have only a provisional response. I have given only the most superficial of looks at the literature. I am mainly tossing out topics for further investigation (hopefully by others!).
But at a glance, I don’t see any obvious reasons to change the judgment of our book that moderate carb consumption, close to the body’s glucose utilization needs, is optimal. In my judgment, “dramatically increasing T3” by eating a high carbohydrate diet (if, indeed, a high-carb diet does this) is probably undesirable. Rather, it’s best to eat a moderate amount of carbohydrate that keeps T3 at physiologically normal, healthy levels. Both too much and too little T3 – and, perhaps, too much and too little dietary carbohydrate – may be dangerous.
Conclusions
In regard to Anthony Colpo, I’d like to extend an olive branch, and reiterate the following points:
- The purpose of Mario’s post last Thursday was not to show that Anthony was right or wrong, but to find out whether we were right or wrong.
- Although we are more sympathetic than Anthony to low-carb diets, we agree that they have risks. Yes, it is possible to become glucose deficient.
- I stand by Mario’s post. I don’t believe there are any errors in it.
- Nothing Mario said contradicted the main points of Anthony’s July 1 post to which it linked. Mario (and we) endorsed “moderate” carb consumption, not very low carb diets, and Mario’s focus on the dangers of high-omega-6 diets should not be construed as a denial of the dangers of very low-carb diets.
Anthony and I exchanged increasingly cordial emails over the weekend, are sending each other copies of our books, and I hope we will be on good terms even if our diet ideas and study interpretations are not identical.
In regard to rT3:T3 ratio, it might be interesting to compile some data on rT3:T3 ratios and carb intake among low-carbers. It may be that studying how rT3:T3 ratio varies with carb consumption will give us a clearer idea of optimal carbohydrate intake. I would expect there would be some “plateau region” of carb intake over which rT3 is low and T3 levels are stable. At very high carb intakes, T3 might be elevated in order to promote glucose disposal; at very low carb intakes, the euthyroid sick syndrome of elevated rT3 and depressed T3 might hold.
Finally, a look at upcoming posts. Yes, long as this post was, we’re not done exploring these issues.
Anthony cited some more papers in his reply to Mario, and Mario will respond in detail: what do those studies prove? The purpose of this is to evaluate our diet to see if our advice is sound, not to feature any disagreements Mario may have with Anthony, or to prove anyone wrong.
In the post after that, we’ll have a fascinating personal story from Gregory Barton. Gregory’s experience connects euthyroid sick syndrome to the vexing issue of high LDL on Paleo diets, and as such ties in with some points Chris Masterjohn has made on the role of thyroid hormone in LDL pathways. As such it may help us reach some closure on two of the outstanding problems that have troubled the low-carb Paleo community.
And if we’re not tired of the issue after those posts, commenter Valtsu has been sending me references to papers discussing links between infections and euthyroid sick syndrome. It looks like toxins and inflammatory cytokines released during infections can disrupt the ability of the hypothalamus to regulate thyroid hormone levels. This could have implications for other diseases besides euthyroid sick syndrome – including obesity, which often features disruption of the hypothalamus’s regulation of energy utilization.
So I think this little controversy is leading us to some productive discoveries. Therefore, I’d like to thank Anthony for raising the issues in the first place. Out of disagreement may come insight.












Recent Comments