Stephan Guyenet recently did a great podcast with Chris Kresser, discussing the relationship between food reward and obesity. At his blog Whole Health Source he has been expanding upon the podcast with a series titled “Food Reward: A Dominant Factor in Obesity.”
Stephan is a neurobiologist and an expert in the role of the brain in obesity – something I know little about – so it was delightful to have a chance to learn from him.
Today I will try to place Stephan’s ideas in a larger context. I will argue that the concept of a fat mass setpoint is best understood as a dynamic equilibrium among many organs of the body, including the brain; and that food reward is a very important factor in the obesity epidemic because it helps explain many aspects of weight gain and loss and explains why many people are so eager to eat toxic and malnourishing foods, but that it may be going too far to call it a “dominant” factor in obesity.
Metabolic Damage
In some ways I think we know about the causes of obesity than about its nature. It’s very easy to induce obesity in both animals and humans: feed a malnourishing diet providing calories in the form of a combination of wheat, fructose, and polyunsaturated fats. The links between these toxic foods and obesity are discussed in our book and in several blog posts (see Why We Get Fat: Food Toxins, Jan 20, 2011, and Wheat and Obesity: More from the China Study, Sep 4, 2010). Malnutrition contributes to obesity by promoting metabolic syndrome and appetite (see Choline Deficiency and Plant Oil Induced Diabetes, Nov 12, 2010).
Grains, fructose sugars, and omega-6 vegetable oils provide about 60% of calories in the modern diet, up from less than 10% in the Paleolithic. The strongest rise has been in omega-6 and fructose consumption since about 1970. At the same time, there has been a shift from home cooking of fresh foods to industrially processed and preserved foods. Lack of freshness and industrial processing can significantly increase food toxicity. With these shifts, obesity rates have skyrocketed.
It seems clear that these toxic, malnourishing diets can induce “metabolic damage”: biological changes that alter the way energy is metabolized and energy expenditure is managed – changes that bring about and maintain obesity.
What Are the Sites of Metabolic Damage?
In the obese, altered biology has been detected in many organs. Examples include:
- Liver
- Adipose tissue
- Brain
- Skeletal muscle
- Gut and gut flora
- Endocrine organs (thyroid, adrenals, pituitary)
Metabolic damage is a complex topic in part because so many parts of the body experience it, and interactions between these organs are crucial to understanding obesity.
Any good theory of obesity will have to explain the damage that occurs in all of these organs. It will also have to explain the interactions and interdependencies among these organs.
Stephan: The Brain’s Sub-Systems Matter
Stephan has taught us an important fact: that the brain has two connected but somewhat independent organs that participate in obesity:
- The energy homeostasis system
- The food-reward system
The energy homeostasis system is located in the hypothalamus and listens to the hormone leptin, which is released by adipose cells. More leptin indicates more fat mass (but not everyone has the same leptin level for the same amount of fat). The energy homeostasis system adjusts activity and thermogenesis (“calories out”) to achieve its desired leptin level – which translates to a desired fat mass “setpoint.”
The food-reward system influences appetite (“calories in”). It evolved for the purpose of getting us to eat the most healthful and beneficial foods. Thus, starches and fats, staples of the Perfect Health Diet, are good at stimulating the food reward system. Eating large amounts of a single flavor is boring; variety – which minimizes the dose of any one toxin, and ensures a diversity of nutrients – is higher in reward.
So one part of the brain manages “calories in” with an eye toward being well nourished, while another part manages “calories out” with an eye toward achieving just the right amount of fat.
What could go wrong?
Misdirected Food Reward
Unfortunately, a reward system that evolved in the Paleolithic is not necessarily a good guide to navigating modern foods:
- New foods have come into existence – agriculturally produced cereal grains, hybridized for greater toxicity; refined fructose-rich sugars; and vegetable seed oils high in omega-6 – that didn’t exist in our evolutionary past. These toxic but malnourishing foods confuse the food reward system by invoking the same signals highly nutritious Paleo foods do – starch; fat; salt – but lack nutritional value, and indeed can act as poisons.
- Food scientists have learned how to design toxic and malnourishing foods that hyperstimulate the food reward system. They stimulate addictive behavior: when you eat one, you want another one, and another. All aspects of the food are designed to trick the food reward system into wanting more – even color.
In this modern environment of industrially processed toxic foods, following our innate food preferences may easily lead us to eat unhealthy diets. It may also lead us to eat more calories than we need, creating a “positive energy balance” that Stephan associates with inflammation.
Food Reward Paradoxes
Food reward is rather hard to make sense of. Many commenters have noted this, and Stephan had to do a post clarifying what he means by food reward:
Food reward is the process by which eating specific foods reinforces behaviors that favor the acquisition and consumption of the food in question. You could also call rewarding food “reinforcing” or “habit-forming”, although not necessarily in an addictive sense.
A seeming paradox is this: On the one hand, the food reward system evolved to guide us toward healthy foods, as Stephan says:
Food reward is essential for survival in a natural environment, because it teaches you what to eat …
Yet in the modern environment eating high-reward foods is supposed to impair health and cause obesity.
This is of course consistent with our view of obesity – modern industrial foods are toxic and malnourishment – but the mechanisms involving the food reward system are still a bit confusing.
One confusing aspect is that Stephan has spoken of the reward value of macronutrients, with carbs and fat being generally more rewarding than protein, and a carb-fat mix being most rewarding.
This does explain certain observed facts: that “lean meat and vegetables” diets, which are high in protein and therefore low in food reward, tend to induce immediate weight loss. Many popular diet books – Atkins, the Eades Protein Power books, the Dukan Diet – recommend such diets; immediate weight loss helps the diets go viral.
Yet it is not clear that it is consistent with all the facts. In particular high food reward may be consistent with good or ill health, obesity or slenderness. Some of the healthiest weight loss diets, such as ours, are high in food reward (see Low-Protein Leanness, Melanesians, and Hara Hachi Bu, Jan 27, 2011; Perfect Health Diet: Weight Loss Version, Feb 1, 2011).
The food reward system evolved to make us healthier. So it would seem to be the modern environment, especially newly available types of high-reward but unhealthy food, that is the cause of obesity. Food reward enters into obesity only because the food reward system no longer guides us to the optimal foods.
On our view, that toxicity is what matters most, the combination of wheat and fructose with polyunsaturated fats creates obesity, while the combination of safe starches with saturated and monounsaturated fats makes one slender. Yet both may have the same proportions of carb and fat! So it is not clear why food reward is a “dominant factor in obesity” if obesity-causing and obesity-curing diets may have similar food reward.
One possible explanation is that food reward is strongly influenced by subtle changes in the intensity of flavors and flavor associations. Seth Roberts today has a post illustrating this: a reader lost almost all excess weight simply by shifting from Coke and Pepsi to iced tea flavored with a cup of sugar per gallon. Seth writes:
His drink was pleasant enough. It derived pleasure from flavor (tea), sweetness (sugar), and sourness (lemon juice).
Of course Coca-Cola is flavored, sweet, and acidic. Why does one drink cause weight loss and the other obesity?
Seth’s correspondent had drank the iced tea daily for 3 years. If rewarding food is food that people keep returning to, then it seems the iced tea was as rewarding as the Coca-Cola. On the other hand, if the only way we have to judge that iced tea is low in food reward is that it leads to weight loss, or that Coca-Cola is high in food reward is that it leads to weight gain, then the theory becomes circular. Is there some independent way of judging food reward?
Toward a Food-Reward Theory of Obesity
To expand this into a theory of obesity, one has to address both the “calories in” and “calories out” sides of the equation; and also the “body composition” issue – if you have more calories in than out, where do they go? To fat or muscle?
Food reward obviously influences “calories in.” But to be “a dominant factor in obesity” the food reward system has to influence “calories out” as well. How does it do this?
Stephan believes that there is “reciprocal regulation” between the food reward system and the brain’s energy homeostasis system, so that when highly rewarding food is available the food reward system persuades the hypothalamus to accept a higher fat mass.
I’m not aware that Stephan has indicated whether he thinks the food reward system can have any influence on body composition.
So the food-reward theory of obesity seems to be only a partial explanation of obesity. Yet there is evidence for it.
Evidence: Weight Plateaus
The greatest merit of the theory is that it explains why weight tends to reach plateaus and stay at specific weights as long as the diet remains unchanged.
I’ve previously shown this plot from Seth Roberts:
Note how every time he adopted a new diet or lifestyle, weight changed rapidly at first and then settled at a plateau. On low-carb, Alex lost 50 pounds in his first year and then spent most of 2003 at 200 pounds with little change. On the Shangri-La diet he lost 30 pounds in six months and then spent a year at a plateau of 190 pounds. A vegan diet moved him to a plateau at 230 pounds, where he seems to have spent about 8 months.
This is exactly what the food-reward theory predicts. A diet stimulates the food-reward system and leads to setting of the fat mass setpoint. Different food rewards, different setpoints. Manipulating food reward, as in Shangri-La Diet or low-carb high-protein dieting, lowers the setpoint.
But here are two things to consider:
(1) There is no evidence that the setpoint that is ultimately reached is the optimal weight. Often the plateau weight is still abnormally high, even on low-carb Paleo or Shangri-La Diets.
(2) There is no evidence that reaching a “normal” weight through a low food reward diet is the same as achieving health.
I think we have to ask the question: what is our goal? Is it weight loss, or is it returning to optimal health? If the latter, does a diet that achieves weight loss by manipulating food reward improve health?
This issue came up in the podcast and Stephan’s answer was that a low food reward diet reduces calorie intake leading to negative or neutral energy balance. In many studies, positive energy balance is associated with increasing inflammation while calorie restriction is associated with improved biomarkers and reduced inflammation. So low food reward diets may well be health improving.
I think this quite likely, but here are two possible objections:
(1) Low food reward dieting has transient and reversible benefits. The period of positive or negative energy balance is transient on all diets; eventually weight settles at a plateau and neutral energy balance is once again attained. So if energy balance is all that matters, the health benefits of a low food reward diet will also be transient. If the low food reward diet is not maintained for life, then eventually a switch to a higher food reward diet will introduce a period of health damage that may exactly compensate for the benefits won during the transition to the low food reward plateau.
(2) Low food reward dieting is suboptimal for health. If food reward evolved to lead us to the healthiest diet in the evolutionary milieu, isn’t the best health to be achieved by eating for HIGH food reward and living in the evolutionary style eating evolutionary foods?
The first issue tells us that for real health benefits, the low food reward diet has to be a lifelong practice, or else there has to be an independent effect of fat mass on health, with elevated fat mass impairing health regardless of energy balance.
The second issue is particularly interesting in light of the fact that some aspects of Stephan’s diet, which he describes in his podcast with Chris Kresser, seem designed to reduce the food reward of his diet. For instance, he minimizes spices or salt, and avoids between-meal snacks.
Salt is a source of food reward. It also may improve health, as it seemed to do in the recent study published in the Journal of the American Medical Association in which people eating 6 g/day (highest third of salt consumption) were only one-fifth as likely to die of heart disease as people eating less than 2.5 g/day (lowest third).
So should we target low food reward, or high food reward but with evolutionary foods in an evolutionary lifestyle?
Our weight loss advice (See Perfect Health Diet: Weight Loss Version, Feb 1, 2011) is essentially the latter. We favor a mixed carb and fat diet with savory sauces and broths that includes high food-reward items like salt. We disagree with the “lean meat and vegetables” approach to weight loss dieting, although we acknowledge that it usually brings on rapid initial weight loss.
I should say that Stephan’s diet, described in the podcast, is very close to ours. It could be described as a high-carb version of the Perfect Health Diet, with diversity of carbs increased by using detoxification procedures like soaking, sprouting, and fermenting to increase the number of “safe starches.” In describing his diet, he mentioned eating rice but not other cereal grains.
So it seems the differences between Stephan’s diet and ours are rather subtle. But one could argue that the differences between the sugared iced tea and Coca-Cola which Seth’s correspondent drank are also subtle. In the food reward theory of obesity, little changes in flavor can make a big difference in weight.
Does Food Reward Explain Obesity – Or Weight?
I think it’s important to distinguish between the disease of obesity – the health disorder characterized by metabolic damage – and the condition of being fat. Consider:
- An obese person whose metabolic damage was suddenly and completely cured would be healthy but still fat, because it would take some time to lose weight. But the weight would fall off rapidly.
- One could be slender and yet still have the disease of obesity, if metabolic damage persisted. If the site of metabolic damage is elsewhere than adipose cells, then liposuction might make a person slender but it wouldn’t cure obesity. The weight would return. This is in fact what happens.
Looking back at Alex Chernavsky’s weight chart, it’s clear that low-carb and Shangri-La diets reduced his weight. It’s not obvious that any diet cured his obesity.
Likewise, we’re all familiar with young people who eat massive quantities of junk food and remain slender. The high food reward diets, even toxic and malnourishing diets, seem not to cause weight gain until some kind of metabolic damage occurs.
It seems that metabolic damage – the disease of obesity – is a prerequisite for food reward to matter.
Stabby found an interesting paper that addresses this. They write:
Only some of the leptin-resistance models (leptin antagonist blockade and aged obese rats) exhibit heightened weight and adiposity gain on a chow diet, while all models discussed demonstrate obesity in the presence of an HF diet. Thus, the leptin resistance appears to be reinforcing “reward eating” beyond caloric energy requirements….
Leptin receptors … act through the JAK-STAT signaling pathway and decrease food consumption upon leptin action. The fact that a chronic reduction in leptin receptor activity in the VTA by siRNA knockdown enhances sensitivity to highly palatable food underscores an important role of leptin receptor function in the regulation of reward feeding behavior (24).
In other words, leptin resistance may have to exist before high-reward foods induce “reward feeding behavior,” or excessive consumption of calories. Likely it has to exist also before the fat mass setpoint is altered from normal.
If obesity (the disease) must exist before food reward becomes a factor in obesity, then it hardly seems likely that food reward is a dominant factor in obesity the disease. It is rather a dominant factor in how much an obese person weighs. That is a different thing.
Fat Mass Setpoint as a Dynamic Equilibrium
Early in this post I listed a half dozen sites of metabolic damage; the brain was only one. I believe that the fat mass setpoint is not controlled by any one metabolic organ, but rather that it is a dynamic equilibrium that is influenced by the whole body.
In other words: metabolic damage anywhere will affect the fat mass setpoint. The brain is not unique in its metabolic role. There are a myriad of ways to alter the fat mass setpoint, and they don’t all involve food, the food reward system, or even the brain.
Is the Brain the Pre-Eminent Site of Metabolic Damage?
Food reward looks to be important because changing the food reward of the diet changes the fat mass setpoint. Reduce food reward and weight drops; raise food reward and weight (usually) increases. This occurs in humans as well as lab animals, as Alex Chernavsky’s chart shows.
But all this really shows us is that food reward is a lever that we can use to adjust weight. It doesn’t tell us that it is the only or most important lever.
Food reward is a very easy lever for scientists to manipulate. It’s easy to replace rodent chow with Cheetos and see what happens. It’s a bit harder to adjust the state of the liver, the adipose tissue, the thyroid, or skeletal muscle.
When those other sites of metabolic damage are manipulated, does the fat mass setpoint change as dramatically as it does when food reward is manipulated?
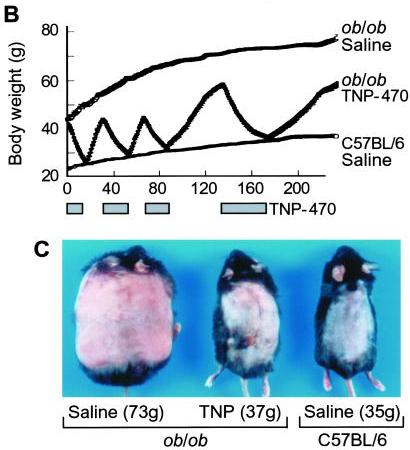
I think it does. Consider this classic study by Maria Rupnick and colleagues. Giving or withholding angiogenesis inhibitors causes mice to cycle between obese and normal weight:
In some ways this weight cycling is more dramatic than any of the food reward studies, because weight in leptin-impaired (ob/ob) mice goes all the way back to normal with angiogenesis inhibition. And it is thought that the angiogenesis is occurring purely in adipose tissue, not in the brain – so it would seem that this is a clean manipulation of adipose tissue only. Perhaps adipose tissue angiogenesis is a “dominant factor in obesity.”
Many other manipulations of adipose tissue change the equilibrium weight (the “fat mass setpoint”). For instance:
- The level of activation of PPAR-gamma affects the amount of leptin released per unit fat mass. PPAR-gamma deficiency leads to hypersecretion of leptin from adipocytes; mice become very slender and adipocytes very small because the brain thinks the body is fat. These mice never develop insulin resistance. PPAR-gamma can be influenced by diet.
- The number of eosinophils – a type of white blood cells – controls whether adipose tissue macrophages are in a pro-inflammatory or anti-inflammatory state. This in turn controls whether adipose cells are insulin resistant or insulin sensitive, with implications for obesity and diabetes. This is one possible pathway by which gut flora may affect obesity, since the types of gut flora influence eosinophil counts.
It’s starting to look like metabolic damage in the adipose tissue alone may be sufficient to induce obesity.
We’ve previously discussed the fact that choline deficiency induces obesity, primarily (it is thought) through effects in the liver. It is likely that alternating high-choline and zero-choline diets would induce fluctuations similar to those in Maria Rupnick’s angiogenic mice. Choline deficiency induced obesity suggests that metabolic damage to the liver alone may be sufficient to induce obesity.
It may be that every organ with a role in metabolic regulation can be manipulated in some way to induce obesity. It looks like the fat mass setpoint is a dynamic equilibrium which depends on the state of every one of the organs involved in metabolic regulation.
If every site of metabolic damage matters and is influential on weight, then it would seem an exaggeration to describe food reward as a dominant factor in obesity. It is an important factor, but not obviously more important than any of the other systems or organs involved in metabolic regulation.
Conclusion
I am grateful to Stephan for sharing his knowledge of the food reward system and the neurobiology of obesity. I immensely enjoyed listening to his podcast and reading his blog posts, and look forward even more to future posts so I can chase references.
But nothing he said has caused me to change my views of obesity or of the best weight loss diet:
- I think the focus should be on recovering health by curing metabolic damage.
- I think our evolved preference for tasty foods including starches, fat, salt, and other “high reward” flavors indicates they are healthy, and therefore that a diet rich in such foods is most likely to cure metabolic damage.
- I think it is essential to stay away from toxic, malnourishing foods made from wheat, fructose sugars, omega-6 oils, and bioactive compounds like MSG; and instead to eat foods that accord with our evolutionary history.
In one sense I think Stephan is right to call food reward a dominant factor in the obesity epidemic. If industrial food designers weren’t trying to make toxic foods rewarding, we might not have an obesity epidemic. People consume large quantities of toxic malnourishing foods because industrial food designers have learned how to conceal their poor taste and make them hyperstimulate our food reward system.
But looking at biology, I have a hard time believing that the food reward system in the brain is a dominant site of metabolic damage. The liver, adipose tissue, and hypothalamus seem likelier candidates to me. So if your goal is not merely to manipulate weight, but to cure the disease of obesity, then I think it is necessary to look not at food reward, but at food toxicity, nutrition, chronic infections, and gut flora. Those are the levers the obese should look to for a cure.













Recent Comments