Erich asked about the link between omega-6 fats and obesity. It’s a good question and also a good way to introduce the first step of the Perfect Health Diet weight loss program: removal of toxic foods from the diet.
Vegetable Oils With Fructose or Alcohol
These toxic foods are particularly dangerous in combination. We discuss this mix of toxins in the book (pp 56-59).
If you feed lab animals high doses of polyunsaturated fat (either omega-6 or omega-3 will do) along with high doses of either fructose or alcohol, then fatty liver disease develops along with metabolic syndrome. Metabolic syndrome is a major risk factor for obesity, and it’s not very difficult to induce obesity on these diets.
Both sugar and vegetable oils are individually risks for obesity:
- Stephan did a nice post a few years back, “Vegetable Oil and Weight Gain,” discussing a couple of studies showing that both rats and humans get fatter the more polyunsaturated fat they eat.
- Dr. Richard Johnson and colleagues did a review of the evidence for sugar (fructose) as a cause of obesity in the American Journal of Clinical Nutrition a few years ago. [1]
What the animal studies show us is that when fructose and vegetable oils are consumed together, they multiply each other’s obesity-inducing effects.
Here are a few pictures illustrating the correlation between polyunsaturated fat consumption, fructose consumption, and obesity.
Here is the Johnson et al chart showing historical fructose consumption in the UK and US [1]:

Here is Stephan’s chart showing historical polyunsaturated fat consumption in the US:
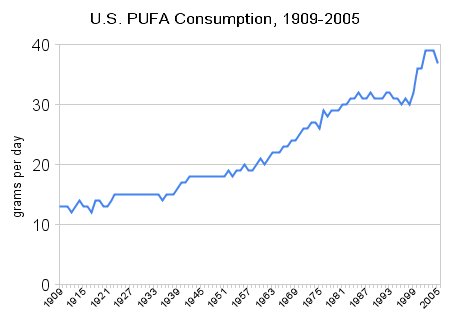
And here are obesity rates in the US:

Cereal Grains
It’s a common observation that the toxic grains, especially wheat, can produce a potbelly or “beer belly.” Rice doesn’t seem to do that.
There is epidemiological evidence for this effect. Here, for instance, is obesity prevalence by country from the World Health Organization Global Infobase:
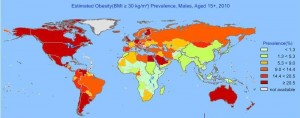
Note the low obesity prevalence in the rice eating countries of China, India, Japan, Indonesia, and southeast Asia; and in sub-Saharan Africa, where a diversity of starch sources are eaten, including manioc/cassava, sorghum, millet, rice, maize, and wheat. The highest obesity prevalence is found in wheat-eating countries.
This correlation persists within countries. In the China Study, the correlation of wheat consumption with BMI was 56%, whereas the correlation of total calorie intake with BMI was only 13%. (Since total calorie intake is correlated with muscle mass, total calorie intake may be completely uncorrelated with fat mass. It’s not how much you eat, but how much wheat!)
Similar outcomes occur in mice. I can’t find any mouse studies comparing wheat to rice, but I did find one comparing wheat to rye [4]. Wheat was far more obesity-inducing than rye:
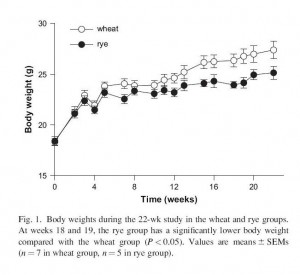
Body fat percentage was 20.2% in the wheat group, 13.7% in the rye group; fasting insulin was 126 pM in the wheat group, 90 pM in the rye group; and fasting cholesterol, triglycerides, and free fatty acids were higher in the wheat group.
In short: wheat made mice fatter, more insulin resistant, and more dyslipidemic than rye.
Just for fun here’s a picture comparing fat tissue in the rye (left) versus wheat (right) fed mice:

I believe that rice would have done even better than rye, but I was unable to find a paper directly comparing rice vs wheat or rye.
Why We Get Fat
This brings me to a point of difference with Gary Taubes. Although glucose is toxic in high doses, the body has an extensive machinery for disposing of excess glucose. As we discussed in our last post, all tissues of the body participate in glucose disposal. Dietary glucose is not likely to do much damage unless the body’s glucose-disposal machinery has been damaged by other toxins first.
Obesity is caused not by carb calories per se, but by natural plant toxins. Plants, not carbs, make you fat!
It’s possible, by the way, that differing toxicities among grains could be responsible for epidemiological evidence favoring “whole grains” over “refined grains.” In America, products made with refined grains are usually 100% wheat; but products made with whole grains are often of mixed origin (“7 grain bread”). Since wheat is the most obesity-inducing grain, dilution of wheat content may be masking the toxicity of whole grains.
Conclusion
Certain toxic foods seem to be very effective at causing obesity: vegetable oils, fructose, and wheat. Along with malnourishment (for instance, by choline deficiency) and infectious disease, food toxins are why we get fat.
The first step in any weight loss effort, therefore, ought to be removal of these toxic foods from the diet. Removing these toxins may not cure obesity; but without this step a cure is unlikely.
References
[1] Johnson RJ et al. Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am J Clin Nutr. 2007 Oct;86(4):899-906. http://pmid.us/17921363.
[2] Andersson U et al. Metabolic effects of whole grain wheat and whole grain rye in the C57BL/6J mouse. Nutrition. 2010 Feb;26(2):230-9. http://pmid.us/19647415.












Recent Comments