So I thought I’d finish up the series on DHA and angiogenesis by discussing 2 issues:
1. First, an assertion: The pathway by which oxidized DHA drives angiogenesis may be really important for human health.
2. Second, the $64,000 question: Is there evidence that high levels of dietary DHA promotes diseases of pathological angiogenesis? What about other dietary factors bearing on DHA oxidation?
Significance of the Oxidized DHA Link to Angiogenesis
The papers discussed in Friday’s post about a major angiogenesis pathway stimulated by oxidized DHA (Omega-3s, Angiogenesis and Cancer: Part II, April 29, 2011) may not seem important to many readers. But to cancer researchers and pharmaceutical companies, this is blockbuster work.
A tumor is, in the words of Hal Dvorak, “a wound that never heals.” [1] To support growth, cancers invoke the wound healing process – especially, creation of new blood vessels, or angiogenesis. But the tumor prevents the wound healing process from completing. If it ever did complete, then the tumor itself would be healed. It would cease to grow and become benign.
It’s been recognized for decades that an ability to block angiogenesis would effectively constitute a cure for cancer. The William Li video explains why: nearly everyone gets microscopic tumors that never develop the ability to induce angiogenesis. Life-threatening cancer is the result of tumors that can induce angiogenesis. No angiogenesis, and no one would die of cancer.
But existing anti-angiogenic cancer therapies have produced disappointing results. Avastin, an anti-angiogenic drug targeting VEGF (vascular endothelial growth factor), has been estimated to extend colon cancer patient lifespan by only 6 weeks. (Nevertheless, Avastin generated $7.3 billion in revenue last year. Imagine how much money there would be in an anti-angiogenic therapy that worked!)
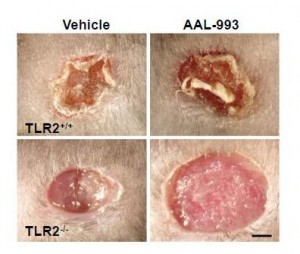
The work I discussed last Friday suggests a reason for that failure. Recall these pictures:
If only the VEGF pathway is blocked (upper right), there is almost as much angiogenesis and wound healing as in a normal wound (upper left). But when both the VEGF and TLR-2 angiogenic pathways are blocked (lower right), there is no wound healing.
If these are the operative pathways in cancer also, then blocking the TLR-2 angiogenesis pathway might be the key to cancer therapy.
But cancer is not the only disease of pathological angiogenesis. Others include:
- Age-related macular degeneration, diabetic retinopathy, and retinopathy of prematurity – three common causes of blindness.
- Atherosclerosis, which often features angiogenic vessels in thickened arterial walls.
- Vascular malformations and tumors.
- Obesity. Adipose tissue utilizes angiogenic pathways, and angiogenesis inhibition prevents the deposition of fat.
- Rosacea, psoriasis, and some other skin conditions.
- Endometriosis, uterine fibroids, and some other causes of female infertility.
- Rheumatoid arthritis.
- Crohn’s disease.
- Preeclampsia.
It may be that the TLR-2 pathway is key to these diseases as well, and that a treatment that inhibits this pathway can cure or improve all of these diseases.
Add up the size of these markets and a pharmaceutical company executive would swoon.
Luckily, we’re not pharmaceutical company executives. But we can still get excited over possibilities to improve these diseases through diet and anti-microbial medicine.
Infections as Contributing Causes of These Diseases
TLR-2 is stimulated by other things besides oxidized DHA. In particular, TLR-2 is an immune molecule which is stimulated by pathogen proteins. As Wikipedia notes:
TLR-2 recognizes many bacterial, fungal, viral, and certain endogenous substances.
This tells us that many pathogens may stimulate angiogenesis through the TLR-2 pathway. As a result, anti-microbial medicines might help treat some diseases of pathological angiogenesis.
Some antibiotics, including doxycycline and minocycline, are known to exercise anti-angiogenic effects independent of the antibiotic effects. [2]
Diet-Induced Angiogenesis
Many foods affect angiogenesis. In fact, cancer studies have identified dozens of plant foods, from garlic to tomatoes to leeks, that possess anti-angiogenic properties.
However, foods can also promote angiogenesis. Let’s stick to the oxidized DHA pathway and see if there’s evidence that foods drive it.
You’ll recall the recipe was:
If this pathway is important in human disease, then we should expect diseases of angiogenesis to be worsened by adding the ingredients on the left.
Specifically, cancer, AMD, rosacea, and so forth should be worsened by high doses of DHA, high doses of vitamin A, and low doses of antioxidant minerals like zinc or selenium.
Is there any evidence for that pattern?
Cancer Studies
First, let me give my bottom line on the Brasky study that kicked off this series. High tissue levels of DHA were associated with increased risk of high-grade prostate cancer, and the oxidized DHA angiogenesis pathway provides a mechanism for this association. What’s not clear is why tissue DHA levels were high. EPA levels were also elevated in the high-grade prostate cancers, but not by nearly as much as DHA levels. EPA and DHA appear together in fish and fish oil, so this suggests that fish consumption contributed to but was not the primary cause of the elevated tissue DHA. The drug finasteride greatly raised risk of high-grade prostate cancer, but the paper did not break down the DHA-cancer association between the finasteride and placebo arms. The most likely explanation, in my view, is that finasteride increases conversion of EPA to DHA and creates artificially high tissue DHA levels. The high DHA levels combined with oxidative stress drive cancer through the TLR-2 angiogenesis pathway.
A clever but unlikely alternative explanation was suggested by Peter at Hyperlipid: perhaps extra dietary fish oil raises testosterone levels. Prostate cancer is a hormone-dependent cancer and can be promoted by testosterone, just as breast cancer is promoted by estrogen. Possible supporting evidence comes from a paper showing an inverse association between metabolic syndrome / diabetes and prostate cancer. The trouble with this idea is that (a) this effect should have been strongest in the low-grade cancers, since diabetes reduced the incidence of low-grade cancers, but in the Brasky study DHA had no association with low-grade cancers, (b) fish oil lowers testosterone levels in rats, (c) in the Brasky study high-grade prostate cancers were strongly associated with obesity and the obese generally have low testosterone levels, and (d) surprisingly, high-grade prostate cancers are associated with low testosterone, not high. So one could argue that fish oil might promote high-grade prostate cancer by lowering testosterone!
A unified explanation along this line would be: Finasteride raises DHA levels, and DHA lowers testosterone. Low testosterone reduces incidence of low-grade prostate cancers but makes it much more likely they will progress to high-grade. Thus, finasteride reduces prostate cancer incidence but increases high-grade prostate cancer incidence and overall prostate cancer mortality. Fits all the facts. Could be.
My bottom line: the Brasky study is weak evidence for anything, but it does raise a whiff of evidence that high dietary fish oil intake might encourage a transition from low-grade to high-grade cancer.
What about other ingredients in the recipe? Does increasing retinyl levels raise cancer risk?
Retinyl palmitate (vitamin A) has been tested in clinical trials for its effect on cancer risk. The trials had to be cut short when it was found that vitamin A increased cancer mortality:
The Carotene and Retinol Efficacy Trial (CARET) was a multicenter randomized, double-blind placebo-controlled chemoprevention trial testing whether daily supplementation with 30 mg β-carotene + 25,000 IU retinyl palmitate would reduce lung cancer risk among 18,314 heavy smokers, former heavy smokers and asbestos-exposed workers. The intervention ended 21 months early in January, 1996 when interim analysis found evidence that the supplements increased the risk of lung cancer and total mortality in this high-risk population by 28% and 17%, respectively (10). [3]
After the study ended participants were tracked for years afterward. Those who had received vitamin A during the trial, but especially those in the vitamin A arm who took additional supplements (mainly multivitamins which are rich in A, but possibly also fish oil), had more high-grade prostate cancers:
As a proportion of the total prostate cancer cases, more men who were randomized to the active arm developed high-grade prostate cancer (Gleason 7-10) than in the placebo arm (44.6% vs. 40.1%, respectively)….
For aggressive prostate cancer, men in the CARET intervention arm who used additional supplements had a relative risk for aggressive prostate cancer (Gleason >or=7 or stage III/IV) of 1.52 (95% CI, 1.03-2.24; P < 0.05), relative to all others. [3]
Interestingly, in the placebo arm taking multivitamins and other supplements reduced cancer risk.
Other studies have found similar results.
Men with higher retinol concentrations at baseline were more likely to develop prostate cancer (quintile 5 vs. quintile 1 hazard ratio = 1.19, 95% confidence interval: 1.03, 1.36; P(trend) = 0.009). The results were similar for aggressive disease. Joint categorization based on baseline and 3-year retinol levels showed that men who were in the highest quintile at both time points had the greatest increased risk (baseline/3-year quintile 5/quintile 5 vs. quintile 1/quintile 1 hazard ratio = 1.31, 95% confidence interval: 1.08, 1.59). In this largest study to date of vitamin A status and subsequent risk of prostate cancer, higher serum retinol was associated with elevated risk, with sustained high exposure conferring the greatest risk. [4]
Carotenoids, which can generally be converted to vitamin A, are also associated with higher cancer risk. There is one exception – lycopene:
Lycopene was inversely associated with prostate cancer risk (comparing highest with lowest quartiles, odds ratio (OR) = 0.65, 95% confidence interval (CI): 0.36, 1.15; test for trend, p = 0.09), particularly for aggressive disease (comparing extreme quartiles, OR = 0.37, 95% CI: 0.15, 0.94; test for trend, p = 0.04). Other carotenoids were positively associated with risk. [5]
What’s special about lycopene? Wikipedia explains:
Lycopene may be the most powerful carotenoid quencher of singlet oxygen,[18] being 100 times more efficient in test tube studies of singlet-oxygen quenching action than vitamin E … The absence of the beta-ionone ring structure for lycopene increases its antioxidant action….
Lycopene is not modified to vitamin A in the body …
So lycopene does not increase retinyl levels, but does act as an extraordinarily powerful antioxidant, thus reducing oxidative stress! If you wanted a good food for stopping the DHA – angiogenesis pathway, you’ve found it: tomatoes.
Hmmm, tomatoes go well with salmon …
That gets us to the third part of the recipe, oxidative stress. If oxidized DHA drives angiogenesis, then antioxidants should be preventative for these diseases.
The evidence here is rather mixed, because with the exception of the negative effects of vitamin A, most antioxidants seem to have little effect on cancer. Nevertheless, I’ll give some studies. Selenium is a antioxidant mineral due to its role in glutathione peroxidase:
Serum selenium was inversely associated with risk of prostate cancer (comparing highest to lowest quartiles, OR = 0.71, 95% CI 0.39-1.28; p for trend = 0.11), with similar patterns seen in both blacks and whites. [6]
Zinc is an antioxidant due to its role in zinc-copper superoxide dismutase. Prostate cancer is associated with low tissue levels of zinc. [7, 8] High dietary intake of zinc is associated with lower rates of prostate cancer. [9]
N-acetylcysteine is an antioxidant supplement that is a precursor to glutathione. N-acetylcysteine has been shown to prevent angiogenesis and has been proposed as a likely cancer preventative, but this is as yet untested. [10]
Other Diseases of Angiogenesis
I’ll skip those for now, other than to note that fish oil is a well-known trigger of rosacea. Is it possible that the mechanism is via TLR-2 activation by oxidized DHA?
Conclusion
At the moment there’s some puffs of smoke but no fire. Observational studies weakly link high DHA, high vitamin A, and low antioxidant status to diseases of angiogenesis such as cancer.
This pattern would be consistent with the idea that the natural pathway used in wound healing to trigger angiogenesis – DHA oxidation and combination with retinyl protein to trigger TLR-2 pathways – is also important for cancer progression.
It suggests a strategy of reduced fish oil and vitamin A consumption and increased intake of certain antioxidants (such as lycopene, zinc, selenium, or NAC) may be helpful against cancer.
However, this idea needs testing. No study in animal cancer models has tested this dietary combination.
Given the many proven benefits of moderate amounts of fish oil, I don’t see a reason yet to alter our recommendation that healthy people should eat a pound of fish per week. That said, I do think very high intakes of fish or fish oil are ill advised. And I’m intrigued by the idea that dietary changes may have the potential to play a powerful role in recovery from diseases of angiogenesis such as cancer.
References
[1] Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986 Dec 25;315(26):1650-9. http://pmid.us/3537791.
[2] Yao JS et al. Comparison of doxycycline and minocycline in the inhibition of VEGF-induced smooth muscle cell migration. Neurochem Int. 2007 Feb;50(3):524-30. http://pmid.us/17145119.
[3] Neuhouser ML et al. Dietary supplement use and prostate cancer risk in the Carotene and Retinol Efficacy Trial. Cancer Epidemiol Biomarkers Prev. 2009 Aug;18(8):2202-6. http://pmid.us/19661078.
[4] Mondul AM et al. Serum retinol and risk of prostate cancer. Am J Epidemiol. 2011 Apr 1;173(7):813-21. http://pmid.us/21389041.
[5] Vogt TM et al. Serum lycopene, other serum carotenoids, and risk of prostate cancer in US Blacks and Whites. Am J Epidemiol. 2002 Jun 1;155(11):1023-32. http://pmid.us/12034581.
[6] Vogt TM et al. Serum selenium and risk of prostate cancer in U.S. blacks and whites. Int J Cancer. 2003 Feb 20;103(5):664-70. http://pmid.us/12494476.
[7] Sarafanov AG et al. Prostate cancer outcome and tissue levels of metal ions. Prostate. 2011 Jan 26. doi: 10.1002/pros.21339. [Epub ahead of print] http://pmid.us/21271612.
[8] Costello LC, Franklin RB. Zinc is decreased in prostate cancer: an established relationship of prostate cancer! J Biol Inorg Chem. 2011 Jan;16(1):3-8. http://pmid.us/21140181.
[9] Epstein MM et al. Dietary zinc and prostate cancer survival in a Swedish cohort. Am J Clin Nutr. 2011 Mar;93(3):586-93. http://pmid.us/21228268.
[10] Noonan DM et al. Angiogenesis and cancer prevention: a vision. Recent Results Cancer Res. 2007;174:219-24. http://pmid.us/17302199.











Recent Comments