A lot has happened since we last did a round-up. Here is a sampling of things we’ve found interesting:
[1] Interesting posts: Jamie Scott channeled his inner Staffan Lindeberg and performed the Vanuatu Study: “The Diet and Lifestyle of the People of Vanuatu: Paleo in Paradise”. It has a lot of pictures and descriptions of the Kitava-like Paleo diet and attractive good health of the natives. Unfortunately, fewer Melanesians are eating traditional diets, and we may not be able to observe traditional diets in native populations much longer.
Chris Masterjohn helps us understand Weston A. Price. He also did a fascinating podcast with Chris Kresser on LDL cholesterol: The Healthy Skeptic Podcast Episode 11. Speaking of cholesterol, Ned Kock reported that alcohol increases LDL cholesterol in people with the ApoE e4 allele, but decreases LDL cholesterol in people with the ApoE e2 allele.
In another post, Ned notes that a 6-foot man can be strong and healthy at 145 pounds. The moral: Be yourself; don’t think your body needs to look like someone else’s.
The opportunity to reverse Type 2 diabetes by diet was in the news, because of a UK study (Pubmed, Full text) in which 11 patients experienced normalization of beta cell function and reversal of Type 2 diabetes on a diet of 600 calories per day: 280 carb calories, 200 protein calories, and 120 fat calories per day (plus considerable fat released from adipose tissue). This is a starvation diet, below our safe minimum of 600 carb+protein calories and undoubtedly deficient in micronutrients and complex biological compounds, since it’s almost impossible to be well nourished on less than 1200 calories per day of real food. Indeed, people on the diet felt they were starving:
“It was very tough. I was hungry all the time. It was a starvation diet and food was on your mind all the time,” he said.
Many bloggers commented, including Peter Dobromylskyj, Jenny Ruhl, and Pål Jåbekk.
Melissa McEwen cured a skin condition by getting more vitamin A. Matt Stone offered a Paleo failure story, and linked to some pictures of hypothyroid faces. CarbSane found that selenium cured her insomnia; in the comments Mario argues that selenium may be protecting against metal and halogen toxicity.
Keith Woodford links to research showing that opioid peptides from cow’s milk drank by the mother can enter babies via breast milk and argues “the implications are huge”. Dr. Briffa notes that the artificial sweetener aspartame is converted to formaldehyde, a potent carcinogen, in the body. Chris Kresser discusses why it’s possible to have trouble with coconut milk. In the comments Tony Mach says the biggest BPA exposure comes from handling cash register receipts.
At Angelo Coppola’s “Latest in Paleo” blog, breastfeeding advice from a recovered boob nazi. (I may discuss this post a bit in an upcoming blog post.)
Via The Telegraph, many dishwashers are infected with fungi and deposit potentially dangerous fungal pathogens on plates and utensils. Via Craig Newmark, Top Ten Myths About Introverts.
Tom Naughton reveals that the government issued health warnings against cholesterol in the 1960s at the direction of Lyndon Johnson – who wanted to reduce the price of eggs to improve the inflation statistics!
NBA player Robert Horry leaves a poignant letter to his daughter, who died from a genetic disease.
Finally, for our academic readers, the oldest known journal rejection letter, written to Ptolemaeus in regard to his method for measuring the circumference of the earth.
[2] Don’t sleep on the sofa darling: Thursday’s post called to mind Petula Clark’s great hit:
In this video, recorded live in 2003 in Paris, Petula is 70 years old and looks great. At 78 she’s still performing. I wonder what diet she eats?
(The 1967 studio version can be heard here.)
[3] It’s safe to come out:
[4] Thank you, Pål – and thank you, readers: While we were on vacation, Pål Jåbekk of Ramblings of a Carnivore posted a very nice review of our book: “The As Good Health As Possible Diet”:
I would like there to be one diet book. One book that is constantly updated with new research. It would be The Diet Book. The book that made all other diet books superfluous. The go to place for everyone interested in achieving good health. The only book we would need….
The one book I’ve found that comes closest to being the diet book to end all other diet books is Perfect Health Diet. Had it replaced the official dietary guidelines we might actually be getting somewhere. The Perfect Health Diet book is not a perfect book, nor should it be. I think that some of the composition could be improved as well as the lay out and I would’ve liked to see some statements moderated, but content wise and information wise, Perfect Health Diet appears as a good first draft of a book with the potential to end the need for any more diet books.
I am excitedly looking forward to the second edition.
Pål is one of our favorite bloggers and a perspicacious writer on health, so this is high praise. As he says, our book is a work in progress. We are still learning, and that is why blogging is so much fun. The growth of knowledge is a cooperative process, and we continue to learn from other bloggers and from our readers.
We believe that diet should be a primary therapy for all diseases, and that with a good diet and appropriate antimicrobial therapies nearly all diseases can be cured. It’s exciting therefore to hear from readers, especially sick readers, who apply our ideas. We are grateful to readers who share their experiences with us, whether good or bad. Both successes and failures are educational.
Like Pål, we look forward to a second edition. We aim for the perfect diet, but we know that we have not yet written the perfect book. To achieve excellence, an evolutionary process is usually required. We’re most grateful to all those who apply our ideas and help us refine them.
[5] Another migraine success story: Speaking of reader feedback, it was great to hear from Rebecca Lachance on Facebook:
Just a note of thanks for helping me control migraines/headaches. Ketogenic diet has made an enormous difference in my life. Down from 24 days of headaches in February to only 4 days in June! My M.D. is suffering cognitive dissonance – thrilled with the decrease of headaches, but “suggests” a minimal dose of statins to prevent atherosclerosis – despite an HDL of 99 and TRG of 52. Obviously, I won’t be taking statins!… Thanks again.
We believe that ketogenic diets are probably therapeutic for nearly all neurological diseases, so we hope more people with brain or nerve disorders will try our version of the ketogenic diet.
[6] Don’t rush to your funeral: A Russian woman, wrongly declared dead, woke up at her own funeral and had a heart attack when she realized she was about to be buried alive.
[7] It’s good to supplement magnesium: A study in AJCN found that women in the highest quartile of dietary magnesium had a 37% lower risk of sudden cardiac death, and in the highest quartile of serum magnesium had a 77% lower risk of sudden cardiac death, than women in the top quartiles. In the same issue, a clinical trial found that supplementation of 500 mg/day magnesium was beneficial for obese people.
We recommend supplementing magnesium at 200 mg to 400 mg per day. 500 mg/day is more likely to produce an observable effect in a 4-week trial, but is more than we would recommend for long-term supplementation.
[8] My interview with Cary Nosler’s Wide World of Health: The podcast is available for download here.
[9] Getting Real at Whole Foods: This has been making the rounds, but it’s good enough for one more showing:
Via Melissa McEwen.
[10] O Primitivo on LDL, meat, and mortality: Ricardo (“O Primitivo”) of Canibais e Reis (“Cannibals and Kings” in Portuguese; inspired by Marvin Harris’s book), who was the source of the data discussed in Tuesday’s post, tried to leave a comment there but it had too many links for our spam filter and was lost. Fortunately, he emailed me with some fascinating information.
First, he has compiled a database specifically correlating LDL cholesterol levels to various health conditions. This is a very valuable database and I hope he’ll blog about it before long. LDL levels are highly correlated with total cholesterol, so the results are similar to those in his total cholesterol database, but still interesting.
O Primitivo also sent links to some of his blog posts:
- On a Korean study showing lowest mortality with TC between 211 and 250 mg/dl.
- On a Japanese study showing lowest mortality with TC between 240 and 259 mg/dl.
- A list of 48 studies showing that higher cholesterol levels in the elderly are associated with longevity and lower mortality. (Get the list in PDF.)
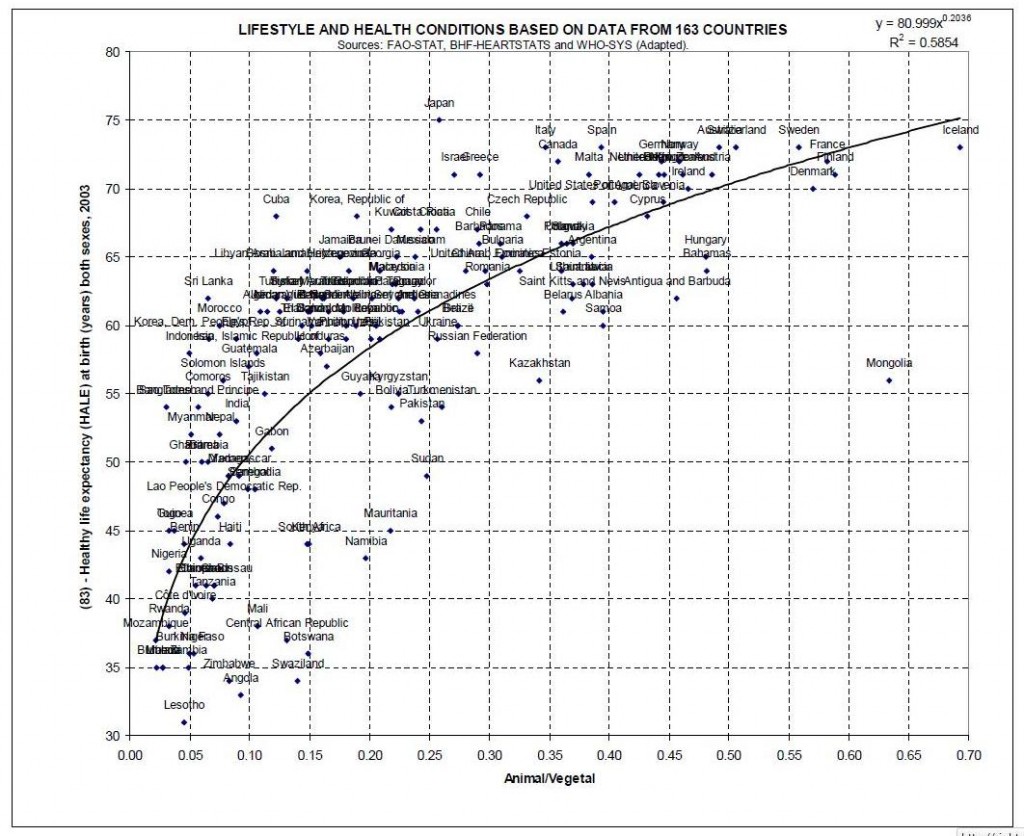
- Statistics on the ratio of animal to plant foods in the diet versus longevity. (In PDF)
- A list of letters and papers from the pioneering cholesterol scholar Dr. Uffe Ravnskov.
Plus a number of links to recent papers which I’ll leave for him to blog about.
Since he has so much good material, and many people will lack time to explore it all, let me give you one highlight. From his document on animal-vegetable ratios, the fraction of food intake from animals versus mortality:
Take that, vegetarians!
Thank you, Ricardo, for all the great information. You have a fantastic blog.
[11] The cat who didn’t bark:
[12] Quote of the week: From a comment by Chris Friederich on Chris Masterjohn’s blog:
“If people let government decide what foods they eat and what medicines they take, their bodies will soon be in as sorry a state as the souls of those who live under tyranny.” – Thomas Jefferson
[13] Who eats better, lab mice or humans?: Paleo bloggers frequently mock scientists for the “Western” diet fed to lab mice: usually some mix of sucrose, casein, and soybean oil. After watching this video, what strikes me about the ingredient lists is that the scientists are right. “Western” humans are eating an awful lot of artificially-colored animal chow:
[14] Race to the bottom continues: Via Bix at Fanatic Cook, a Japanese food scientist has learned how to make “turd burgers” – fake meat made of protein derived from bacteria in sewage, and “improved” by the addition of soy protein:
[15] Shou-Ching’s photo art: Belated Father’s Day edition:
[16] Weekly video: The United States has been losing family farms. One reason is aggressive enforcement of counter-intuitive and health-damaging farm regulations. The new movie “Farmageddon” documents how difficult it is for family farms to produce healthy food. Here is the trailer:
Farmageddon – Movie Trailer from Kristin Canty on Vimeo.
Via Scott Kustes.














Recent Comments