In the January 1 edition of The New York Times Magazine, Tara Parker-Pope’s “The Fat Trap” looks at one of the most interesting aspects of obesity: how difficult it is to keep lost weight from coming back.
I skimmed it when it first came out, but after an email arrived this morning inviting me to sign a petition authored by Gary Taubes, I decided to read it carefully.
Ms. Parker-Pope’s article is excellent. Since it presents valuable evidence on some issues I have been planning to write about, I thought I’d use it to begin expounding my theory of obesity.
The Yo-Yo Dieting Pattern
A common experience on weight loss diets is successful weight loss – but often not to normal weight – followed by unremitting hunger that requires heroic willpower to resist, and ultimate capitulation leading to weight regain. This pattern may repeat itself in yo-yo fashion.
Parker-Pope describes a recent study from The New England Journal of Medicine:
After a year, the patients already had regained an average of 11 of the pounds they struggled so hard to lose. They also reported feeling far more hungry and preoccupied with food than before they lost the weight.
While researchers have known for decades that the body undergoes various metabolic and hormonal changes while it’s losing weight, the Australian team detected something new. A full year after significant weight loss, these men and women remained in what could be described as a biologically altered state. Their still-plump bodies were acting as if they were starving and were working overtime to regain the pounds they lost…. It was almost as if weight loss had put their bodies into a unique metabolic state, a sort of post-dieting syndrome that set them apart from people who hadn’t tried to lose weight in the first place.
The study measured hormonal levels a year after the weight loss:
One year after the initial weight loss, there were still significant differences from baseline in the mean levels of leptin (P<0.001), peptide YY (P<0.001), cholecystokinin (P=0.04), insulin (P=0.01), ghrelin (P<0.001), gastric inhibitory polypeptide (P<0.001), and pancreatic polypeptide (P=0.002), as well as hunger (P<0.001).
Note that insulin levels were still lowered, even as the participants were re-gaining weight:
Decreases in insulin levels after weight loss were evident, and the interaction between postprandial period and study week was significant (P<0.001), with significant reductions in meal-stimulated insulin release 30 and 60 minutes after eating, both from baseline to week 10 (P<0.001 for the two postprandial comparisons) and from baseline to week 62 (P<0.001 for the comparison at 30 minutes; P = 0.01 for the comparison at 60 minutes).
Gary Taubes, in his petition, complains that Ms. Parker-Pope “forgot to mention that the hormone insulin is primarily responsible for storing fat in her fat tissue”; perhaps this omission was just as well.
Resistance to Weight Gain
There is variability in the response to overfeeding. Commenting on a seminal series of experiments published in the 1990s by Canadian researchers Claude Bouchard and Angelo Tremblay, Parker-Pope writes:
That experimental binge should have translated into a weight gain of roughly 24 pounds (based on 3,500 calories to a pound). But some gained less than 10 pounds, while others gained as much as 29 pounds.
Note that eating a pound’s worth of calories typically led to something like a half-pound of weight gain; this shows that weight increases lead to energy expenditure increases. This was in a study in which the subjects were prevented from exercising. Likely the weight gain would have been generally lower if the subjects had been free to move as they wished.
Genes Influence But Don’t Decide
Genes – at least the known ones – are not determinate for obesity:
Recently the British television show “Embarrassing Fat Bodies” asked Frayling’s lab to test for fat-promoting genes, and the results showed one very overweight family had a lower-than-average risk for obesity.
Successful Weight Loss Is Possible
Some people do lose weight successfully:
The National Weight Control Registry tracks 10,000 people who have lost weight and have kept it off. “We set it up in response to comments that nobody ever succeeds at weight loss,” says Rena Wing, a professor of psychiatry and human behavior at Brown University’s Alpert Medical School, who helped create the registry with James O. Hill, director of the Center for Human Nutrition at the University of Colorado at Denver. “We had two goals: to prove there were people who did, and to try to learn from them about what they do to achieve this long-term weight loss.” Anyone who has lost 30 pounds and kept it off for at least a year is eligible to join the study, though the average member has lost 70 pounds and remained at that weight for six years.
Kudos to Drs. Wing and Hill: This is precisely the kind of data-gathering effort that is needed to help us understand weight loss.
The results, at least as reported by the Times piece, aren’t what most dieters want to hear. The people who kept weight off were those who basically continued some form of calorie restriction indefinitely:
There is no consistent pattern to how people in the registry lost weight — some did it on Weight Watchers, others with Jenny Craig, some by cutting carbs on the Atkins diet and a very small number lost weight through surgery. But their eating and exercise habits appear to reflect what researchers find in the lab: to lose weight and keep it off, a person must eat fewer calories and exercise far more than a person who maintains the same weight naturally.
If this is true, then few people have figured out how to cure their obesity. Rather, they’ve just found ways to keep weight off while remaining “metabolically damaged.” They can’t live like normal people and maintain a normal weight.
Paleo Helps
The piece then goes on to discuss the case of Janice and Adam Bridge. Mrs. Bridge peaked at 330 pounds in 2004, now weighs 195; Mr. Bridge peaked at 310 pounds and now weighs 200.
Mrs. Bridge stays at 195 pounds with a reduced-carb Paleo-style diet:
Based on metabolism data she collected from the weight-loss clinic and her own calculations, she has discovered that to keep her current weight of 195 pounds, she can eat 2,000 calories a day as long as she burns 500 calories in exercise. She avoids junk food, bread and pasta and many dairy products and tries to make sure nearly a third of her calories come from protein.
No junk food (presumably sugar), bread, pasta, or dairy is pretty Paleo. Compared to the standard American diet, it’s low in carbs and high in protein.
Persistent Alterations in the Formerly Obese
The article points to other sources of evidence for metabolic differences between the obese and the never-obese.
[O]ne woman who entered the Columbia studies [of Drs Rudolph Leibel and Michael Rosenbaum] at 230 pounds was eating about 3,000 calories to maintain that weight. Once she dropped to 190 pounds, losing 17 percent of her body weight, metabolic studies determined that she needed about 2,300 daily calories to maintain the new lower weight. That may sound like plenty, but the typical 30-year-old 190-pound woman can consume about 2,600 calories to maintain her weight — 300 more calories than the woman who dieted to get there.
Presumably 190 pounds is still obese for the “typical” 30-year-old woman. So the reduced-weight obese woman is burning fewer calories than a same-size obese woman who never reduced her weight.
So obesity followed by a malnourishing weight loss diet often creates persistent changes that hinder further weight loss, or even maintenance of the lower weight. One observation:
Muscle biopsies taken before, during and after weight loss show that once a person drops weight, their muscle fibers undergo a transformation, making them more like highly efficient “slow twitch” muscle fibers. A result is that after losing weight, your muscles burn 20 to 25 percent fewer calories during everyday activity and moderate aerobic exercise than those of a person who is naturally at the same weight.
Another observation in these patients is persistent hunger. Self-reported hunger is confirmed by observable changes in the brain:
After weight loss, when the dieter looked at food, the scans showed a bigger response in the parts of the brain associated with reward and a lower response in the areas associated with control.
In the Columbia patients, the effect is highly persistent:
How long this state lasts isn’t known, but preliminary research at Columbia suggests that for as many as six years after weight loss, the body continues to defend the old, higher weight by burning off far fewer calories than would be expected. The problem could persist indefinitely.
What Caused the Metabolic Alterations?
Are these persistent alterations to the body caused by the original obesity, or by the malnourishing diet that produced the weight loss? Dr. Leibel believes that the cause was the obesity, but that it is slow-acting – requiring an extended period of fatness:
What’s not clear from the research is whether there is a window during which we can gain weight and then lose it without creating biological backlash…. [R]esearchers don’t know how long it takes for the body to reset itself permanently to a higher weight. The good news is that it doesn’t seem to happen overnight.
“For a mouse, I know the time period is somewhere around eight months,” Leibel says. “Before that time, a fat mouse can come back to being a skinny mouse again without too much adjustment. For a human we don’t know, but I’m pretty sure it’s not measured in months, but in years.”
However, other researchers are exploring the possibility that it was the malnourishing weight loss diet that was at fault:
One question many researchers think about is whether losing weight more slowly would make it more sustainable than the fast weight loss often used in scientific studies. Leibel says the pace of weight loss is unlikely to make a difference, because the body’s warning system is based solely on how much fat a person loses, not how quickly he or she loses it. Even so, Proietto is now conducting a study using a slower weight-loss method and following dieters for three years instead of one.
My Theory of Obesity: Lean Tissue Feedback
I’m going to be spelling out my theory of obesity over coming months, but let me introduce here a few hypotheses which can account for the data reported in Ms. Parker-Pope’s article.
I believe the brain defends not only (or primarily) an amount of fat mass, but also the health of the body, as reflected by the quantity and quality of lean tissue.
So it is plausible to speak in terms of set points, but there are two set points: a “fat mass set point”, and a “lean tissue quality set point.” The second is dominant: Lean tissue is essential to life, while gains in fat mass may diminish fitness in some environments but will increase fitness in others and are rarely catastrophic. So the tissue-quality set point usually dominates the fat mass set point in its influence upon the brain and behavior.
Feedback to the brain about the quantity of fat mass comes to the brain through a hormone, leptin, that researchers can easily monitor; but feedback about the state of lean tissue comes through the nerves, which sense the state of tissues throughout the body. Lean tissue is too important for health, and can be degraded in so many different ways, that signals about its state cannot be entrusted to a fragile, low-bandwidth mechanism like a hormone. Lean tissue signaling uses the high-bandwidth communications of the nervous system. This feedback system is hard for researchers to monitor.
So the “fat mass set point” is visible to researchers, but the “lean tissue quality set point” is invisible. This is why researchers focus on the fat mass set point, while actual dieters, who know their own experiences are not explained by a simple fat mass set point theory, resist the idea.
Malnutrition will decrease tissue quality, triggering the brain to increase appetite (to get more nutrients) and diminish resource utilization (to conserve nutrients).
If the diet is deficient in the nutrients needed to build tissue, but rich in calories, then tissue-driven increases in appetite and reductions in nutrient utilization may (not necessarily, because the body has many resources for optimizing lean tissue and fat mass independently) lead to an increase in fat mass. Eventually a rise in leptin counterbalances the tissue-driven signals, but this occurs at a new equilibrium featuring higher fat mass, higher appetite, and reduced nutrient utilization compared to the pre-obese state.
Leptin signaling is responsible for the resistance to fat mass increases. The degree to which this resistance affects outcomes depends on the quality of lean tissue. The higher the quality of lean tissue, the less the brain needs to protect it and the more sensitive it is to leptin. The lower the quality of lean tissue, the more lean-tissue drives dominate and the more the brain ignores leptin signals (is “leptin resistant”).
Malnourishing “starvation” weight loss diets degrade lean tissue, and therefore they make the brain hungrier then it was before the weight loss, more eager to conserve resources that might be useful to lean tissue, and more leptin resistant.
However, weight loss diets that restrict calories, but improve the nourishment of lean tissue, should have the opposite effect. They should make the brain less hungry, less focused on conserving resources, and more leptin sensitive.
How much has to be eaten to provide adequate nourishment to lean tissue? In Perfect Health Diet: Weight Loss Version (Feb 1, 2011), I explored this question. Just to provide the necessary macronutrients to maintain lean tissue, I believe it’s necessary to consume at least 1200 calories per day. To optimize micronutrients as well, it’s probably necessary to supplement, even on a 1200 calorie diet. This is on a perfectly-designed diet. The less nourishing the diet, the more calories will be needed to eliminate tissue-driven hunger.
The Experiences of Perfect Health Dieters
A few Perfect Health Dieters have been using our diet for weight loss for a long enough period of time – 9-12 months – to test this hypothesis.
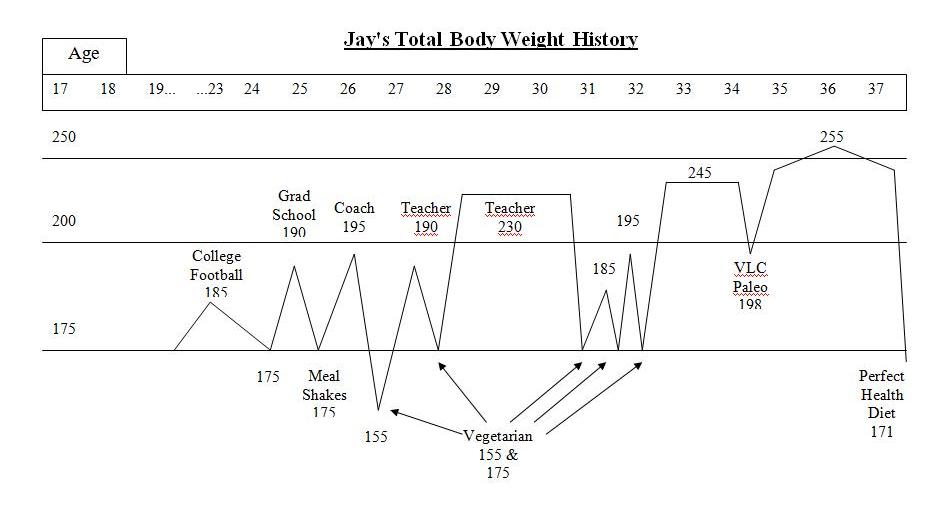
Jay Wright’s Weight Loss Journey (Dec 1, 2011) is a carefully chronicled account. Jay became overweight in college, obese by age 28, and had been obese for 10 years by the time he started our diet. He described his weight loss history:
I was a yo-yo dieter – I could lose weight but it always ended up even higher. I tried meal shake replacements, frozen dinners to limit calories, no meat/meat, no dairy/dairy, acid/alkaline, exercise/no exercise while dieting, no cash or credit cards in my wallet going to work so I wouldn’t stop at a fast food, punishment where I had to eat a raw tomato if I cheat (I hate raw tomatoes), and many other vegetarian leaning and mental tricks. A pattern emerged with these diets. I would starve with low energy for about a week or two until my will power ran out. Then, I would go eat something “bad.” If I continued to repeat the pattern and managed to be “successful,” I stayed hungry even once I reached my goal weight. I tried to transition to a “regular” amount of food to stop starving and just maintain but to no avail. My weight went right back up even higher than before even without cheating on the diets.
This yo-yo pattern of hunger followed by weight regain exactly fits the experiences described in Tara Parker-Pope’s article.
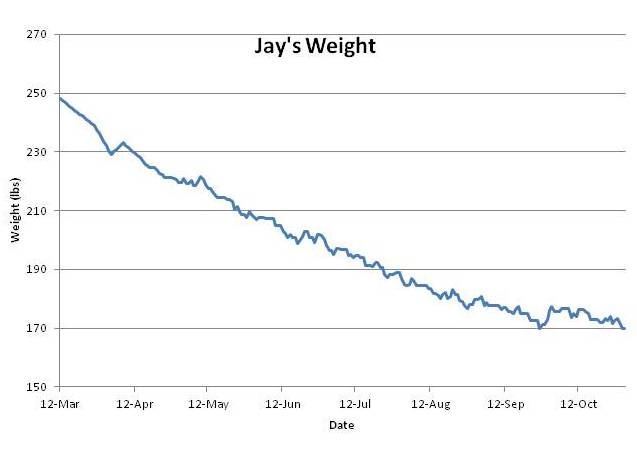
However, Jay’s experience on PHD breaks the pattern. Jay went from 250 pounds to 170 pounds – his normal weight – in six months. Weight loss was steady and he experienced little hunger. He’s maintained his normal weight without regain for 3 months.
This is just as my theory predicts. PHD is a lean-tissue supporting diet, and if his lean tissue is well nourished, he should feel little hunger. If his lean tissue heals fast enough, then his lean-tissue drive will decrease faster than his leptin signaling, his equilibrium weight determined by the balance of these two drives will always be below his actual weight, and he should experience smooth weight loss. Which he did:
Jay’s experience is counter-evidence to many of the ideas put forth by the academic researchers in Ms. Parker-Pope’s article. For instance, Dr. Leibel’s theory that months of obesity create a persistent rise in set point is refuted; Jay had been obese for 10 years but his set point was quickly reset.
Here are Jay’s before and after photos:
Conclusion
I’ll be spelling out my theory of obesity in much more detail later; this is only a first installment.
But I’ll say this: I’ve been gratified by the experiences of people who have tried our diet for weight loss. Our Results page has many reports of reduced hunger, reduced food cravings, and weight loss.
Even those who have not lost weight have reported greatly reduced hunger. I think that means their lean tissue is becoming better nourished, causing the brain to feel less urgency about acquiring more nutrients.
I think this reduction in hunger is the proper first step to healthy weight loss. And I hope that in time we can gather enough case studies to prove that a nourishing diet like the Perfect Health Diet is the best approach to weight loss — and to a genuine cure for obesity.












@Martin Levac
“If injecting insulin locally has this effect locally, then secreting insulin systemically should have the same effect systemically too.”
A logical deduction but not true. Health and wellness people/”gurus” do this all of the time-often basing entire systems on these false deductions. Average every day people (people who don’t study this stuff at any length) don’t know any better and get bamboozled easily.
@Steph,
“Reasonable calories” is totally subjective and individual, as is people’s idea of normal. “Normal” to me might be how my friend, who appears to eat as much as he wants and maintain weight (which isn’t true), eats. “Normal” to someone else might mean eating Hamburger Helper every night because it’s cheap and they have four kids to feed.
PHD may help alter your maintenance calories upward, but it will likely take more than that, like increasing general activity or exercise. Also, I believe (no evidence, however) that the longer you can maintain a certain weight, the more likely your body is to re-set at that weight. So eating what it takes to stay there becomes easier over time, although possibly more difficult in the beginning (when most people quit-then claiming that calorie restriction doesn’t work).
Paul
3 months weight loss maintenance is interesting but not convincing. Here’s the comment from a friend of mine, who lost weight in preparation for bariatric surgery. He and peers in a support group have all had problem maintaining the loss:
‘I take the point, but I do think there is something chemical going on, as well as psychological, when you go through significant weight loss. I feel – as do many of my “peers” – that it is like having a period of grace post surgery, for a year, when eating doesn’t interest you, and then it feels like a switch has been turned back on.’
So show us Jay’s results 2 years on and I’ll be impressed.
Suzanne
True, Thomas, reasonable is subjective. I wonder if you had twins, one formerly obese, one never obese, now same weight, would they maintain on the same number of calories? Maybe, in time?
Hi Paul,
Great post! Can’t wait to read the rest.
Now, on regarding this observed hysteresis to wait gain/loss, as well as caloric intake, it seems to me the body behaves as expected.
Namely, why would a starved cell (adipocyte) NOT ask for food? AFAIK, when we become obese, more adipocytes get created, but they don’t go away (via apoptosis) when we lose weight. This in itself seems enough to me to explain the observed behavior.
The brain is in charge (via various signals, whatever they are) to sense the body’s needs and allocate nutrients to the most important tissue first. I think this is pretty clear by now, even if we don’t know all the pathways on how it happens.
When we gain a lot of weight, more fats cells get created (and insulin might be involved in this process) for obvious reasons.
Cells normally are like well trained soldiers: give out all relevant information, they listen to commands, even commit suicide if asked to. However, they must retain a certain degree of selfishness, and so they will try to survive if they can, so it is not surprising they don’t go away when we lose weight.
So when calories get restricted, the brain partitions the precious little that it has to the most important (metabolically active) tissue first, and so fat cells will starve. They are obedient and they’ll do so for the greater good, but they will let the brain know that they are starving — so we’ll be hungry. I think this is a feature, not a bug 🙂
Now, when we want to return to normal caloric intake, once the important tissue is fed, the starving adipocytes will say “finally, there’s something for us!”, and start eating. This is why we need to maintain the caloric deficit from normal, so that we force the brain to signal the adipocytes to stay put and not eat, as there’s not enough for the important tissue.
If this story is correct, what can we do? Well:
a. nuke the starving adipocytes! They are crying all the time for more and we now have other interests 🙂 We can do so via liposuction or directing them to commit sepuku.
b. increase the amount of metabolically active tissue so its voice gets stronger. That means weight training. However, we’ll still have to learn to deal with a little bit of constant hunger maybe, but if the signal is low enough we might be able to mask it via diet.
c. learn to deal with a bit of hunger, and realize that’s the price to pay for overeating in the first place.
Regarding (a), I wonder if there’s any data from people doing liposuction — according to this story, they should have an easier time keeping the weight off. Unfortunately I think liposuction would also be behavior modifying and you might not care as much and continue partying hard…
Dimi.
Lots of well known LC petition signees.
Great post Paul,
I’ve got a working hypothesis on this subject as well. It seems like some people are able to eat a pretty fair amount between 10-14% body fat without gaining much weight. However, it seems like you almost have to count calories and cut calories dramatically to get down to 5-7% body fat. Not that this is exactly healthy, but I would bet is has a lot to do with set point and the body fearing a loss of muscle.
Thoughts?
thanks Paul,
-Armi
@Thomas, responding to Martin Levac’s:
‘ “If injecting insulin locally has this effect locally, then secreting insulin systemically should have the same effect systemically too.” ‘
“A logical deduction but not true.”
You must be using a strange definition of logic. By logic we usually mean the rules of inference that invariably lead from true hypotheses to true conclusions, regardless of the subject matter.
So if Martin’s hypothesis is true, yet his conclusion false, his logic must be faulty.
Here, the necessary extra assumption that seems innocuous is that the effect of a hormone is independent of whether it is injected or produced endogenously. And that isn’t always true, perhaps because an endogenously secretion occurs simultaneously with various cofactors or with some adaptation/anticipation or whatever. Perhaps you can tell us what happens with insulin?
Logic helps us find these extra assumptions, so that we can then go on to test tem experimentally. Success in science relies on the combination of logic and experimentation, so please don’t berate logic.
@Ulrik
There is evidence for this effect of insulin on adipocytes proliferation. I invite people to Google “insulin adipocytes proliferation” and select the appropriate papers on the subject, for those who’d like to read more about this.
I do make the assumption that the effect of insulin is the same whether we inject it or we secrete it. There’s evidence that suggests this is true for all hormones as well. Testosterone, growth hormone, leptin, ghrelin, etc. All these have the same effect regarding their respective cellular functions independently of the way they enter the body, through injection or through secretion. Specifically regarding insulin, the same assumption was probably made when the first insulin injection was performed back in the 1920’s to treat diabetics type 1. Though at the time we probably did not anticipate this effect of localized adipocytes proliferation; that took years to show itself.
Furthermore, there is evidence that supports this assumption in the form of a recently discovered drug, Adipotide, which targets and kills the blood vessels supplying white fat cells, thereby causing these starved adipocytes to commit suicide (apoptosis), thereby reducing the number of fat cells in a permanent fashion, and ultimately reducing total fat mass. Though human evidence of this is yet to come, human trials have just started.
The point is that all of this taken together appears to refute the idea that fat mass is “protected” by the brain, and supports the idea that fat mass protects itself all on its own just fine.
@Urlik,
I’m saying that local effects are do not necessarily translate to systemic effects, especially with regard to insulin. So yes, faulty logic (sorry to insult your intelligence, I’ll try harder next time).
@Martin Levac
My comment was directed at Thomas, for two reasons:
One, because I disliked how he used the word “logical” to mean something it doesn’t.
And two, because I wanted him to explain why he thinks your conclusion isn’t true, that is, how come endogenous insulin secretion doesn’t cause adipocyte proliferation, when injections do.
My background is in logic, not in biochemistry, so I don’t claim to know. A few things I’ve heard about hormones though, is that the effects differ between acutely elevated levels and chronically elevated levels of a hormone. Also, when supplementing with testosterone, for instance, the endogenous production is reduced, possibly causing trouble with other hormones (so that’s another case where the exogenous supply is different from the endogenous supply; there are feedback loops that can detect the difference).
For these reasons, I agree with you that talking about “set points” is highly misleading: it conjures up an image of the brain explicitly measuring for instance fat mass and comparing it with a “goal” value, when what’s really happened may be better described as a system of feedback loops with many parts giving homeostasis (or homeodynamics, as the case may be).
@Thomas,
No worries, I’m not easily insulted 🙂
But I still would appreciate your explanation of why the local effects of insulin don’t translate to systemic effects (in regards to adipocyte proliferation, for instance).
@Ulrik
On the subject of acute vs chronic insulin level. That’s an interesting topic, I think.
Based on what I read, when injecting insulin subcutaneously, it is released into the bloodstream very slowly compared to IV injection. This means it stays in the injection spot longer, which suggests that it has a greater effect on that spot. This is supported by the evidence with diabetics type 1 who end up with lipodystrophy in the spots they’ve injected insulin for years. We could describe this as chronic local hyperinsulinemia.
Also, the papers I referred to talk about this difference. According to them, the effect on adipocytes proliferation is not immediate, it takes a few days to be seen. This could explain why injecting insulin has this effect locally, but can still treat diabetics type 1 successfully with the appropriate dosage without otherwise causing excess adipocytes proliferation systemically.
On the subject of logic, I’m with you all the way.
You differentiate between lean tissue signalling and fat tissue signalling. Do you think there is a difference between adipose tissue signalling and all of the other essential fat in the body? Is the brain fatty tissue sending out leptin?
“If injecting insulin locally has this effect locally, then secreting insulin systemically should have the same effect systemically too.”
=================================================
The key word is “injecting” . When someone injects any hormone into the body , there will be an equal and oposite reaction. If you inject any hormone there will be opposite reactions to it.
Since insulin is seen largely as an anabolic, hypoglycemic and anorectic hormone the body will come back with all the stress hormones to restore some sort of a balance. Actually injecting insulin into the brain of animals suppresses hunger and potentiates weight loss through sympathetic system stimulation, quiet unlike the low carb or anti insulin dogma that you come from.
The notion that insulin is a fat regulator is ridiculous to anyone with a base physiological knowledge . Every textbook and study will tell you that insulin is promoting satiety and can not carry fatty acids.
@Vladex,
“The key word is “injecting” . When someone injects any hormone into the body , there will be an equal and oposite reaction. If you inject any hormone there will be opposite reactions to it.”
That’s true. We call this a negative feedback loop. It occurs with testosterone, GH, and thyroid for example. The regulation of these hormones depends in part on the level of these hormones in the blood. Inject one, and endogenous production drops to compensate. This is why injecting testosterone will cause the testes to atrophy for example.
But this negative feedback loop changes nothing to testosterone’s many cellular functions. Inject more testosterone, and we grow bigger muscles. Secrete more testosterone, and we grow bigger muscles too. Inject more GH, and we grow bigger muscles as well as reduce fat mass. Secrete more GH, the same happens. Ingest more thyroid, and metabolism goes up. Secrete more thyroid, the same.
As with these hormones, so it is with insulin. The effect of insulin on its many cellular functions is identical whether injecting or secreting. Insulin is indeed anorectic when injected directly into the brain, it should do the same when secreted by the pancreas, right? And the more we inject, the more anorectic it is, right? So how do we explain the coexistence of obesity and hyperinsulinemia in the same individuals? What causes insulin to stop doing its job in the brain?
To answer that question, we should talk about leptin. Leptin is also an anorectic. It’s supposed to tell the brain when we’re full, when to stop eating. When you inject leptin in a leptin-deficient individual, their hunger returns to normal, and they lose weight. But when you inject leptin in an obese person who already secretes tons of leptin, there is no effect, they remain obese. The problem in this case is leptin resistance. However in both leptin-deficiency and leptin resistance, the real problem is the signal that leptin should send is not heard.
As with leptin, so it is with insulin in the brain. With the obese, in spite of secreting tons of insulin, the signal to the brain that should cause us to stop eating, is not heard. With diabetics type 1, we see the same problem. They don’t secrete insulin, and eat constantly. The signal is not heard by the brain. It’s important to note that in diabetics type 1, the problem is not obesity, it’s emaciation. Injecting insulin in these individuals will cause them to gain weight.
There’s a contradiction here. In leptin-deficient individuals, they eat too much, and gain weight. But in diabetics type 1, they eat too much, yet lose weight. In leptin-deficient, we inject leptin, they eat less, lose weight. In diabetics type 1, we inject insulin, they eat less, yet gain weight.
The point is that we clearly see that what controls both body mass and caloric intake is hormones, not the brain.
When you inject testosterone into someone they lose sex drive, lose hair and get man boobs, exactly opposite of what real endogenous testosterone does in the body.It’s not just that one hormone gets shut down but the opposite regulatory hormones get upregualated. This is obvious as it’s the the body’s way of keeping a ballance.
When you inject insulin into someone , it will upregulate all the hyperglycemic and catabolic hormones and there are many of them to counter insulin
=============================================
The point is that we clearly see that what controls both body mass and caloric intake is hormones, not the brain.
==============================================
all hormones are controlled by the brain. Hypothalamus and all of its associated hormones including insulin controls hunger, satiety and body mass
@Vladex
The effects of exogenous testosterone are identical to endogenous testosterone. The hair loss is due to the effect of dihydrotestosterone, and to genetic predisposition. DHT is synthesized from testosterone through 5a-reductase. The lost of sex drive is due to a negative feedback loop involving estrogen and prolactin. Prolactin is the “satisfaction” hormone. It rises after orgasm. Estrogen is a prolactin agonist. Man boobs is due to estrogen, converted from testosterone through aromatase.
All of the above occurs with both exogenous and endogenous testosterone. However, with exogenous testosterone, we usually inject many times more than endogenous, therefore the effects are that much more severe. But if you produce too much endogenous testosterone, the effects would depend on where the feedback loop broke. If it’s at the aromatase link (aromatase deficiency), then you’ll have problems with your bones due to estrogen deficiency. Treatment for this is exogenous estradiol or estrogen.
All of this is negative feedback loops. It’s got nothing to do with the actual cellular functions of the respective hormones. If you inject estrogen for example, you’ll get man boobs too. The effect of estrogen is identical whether you inject it or convert it from testosterone.
As for the brain controlling all hormones, that’s not true. Insulin for example is regulated by a direct positive feedback loop at the pancreas with blood glucose level. Insulin is also regulated by systemic insulin resistance, which is regulated in part by intra-cellular and liver glucose/glycogen reserves. Insulin resistance basically regulates the rate of disposal of insulin. When insulin resistance goes up, disposal rate goes down, insulin goes up. Leptin is regulated by adipocytes. The more adipocytes, the more leptin. Ghrelin is regulated by the stomach and pancreas. Interestingly, these three hormones regulate hunger, satiety and food intake.
So how does the brain regulate hunger, satiety and food intake? It doesn’t. It merely responds to these hormones, which are regulated primarily by fuel status at the adipocytes, at the pancreas, and at the stomach.
The effects of exogenous testosterone are identical to endogenous testosterone.
=====================================================
If all testosterone is the same , that would mean that teenage boys who have the highest levels of testosterone in life would be losing hair, have no sex drive and devloping man boobs. What matters is the dose obviously and the state of the body. Most if not all people developing those symptoms have low testosterone not high.
Also the same thing for all other hormones that are injected is that they carry side effects as the body tries to get in ballance . Estrogen comes before testosterone which is why T gets converted to E but not vice versa but injecting estrogen has many side effects and much worse that T.
==================================================
As for the brain controlling all hormones, that’s not true. Insulin for example is regulated by a direct positive feedback loop at the pancreas with blood glucose level.
=================================================
Wrong, insulin like all hormones is controlled by the brain. Insulin receptors are all throughout the brain particularly in hypothalamus and olfactory bulb. This is why insulin gets secreted at the sight and particularly smell of food before it even enters the mouth and it prepares the pancreas to secrete it at the expectation of the food. When one eats, insulin sends satiety message to the hypothalamus
================================================
Ghrelin is regulated by the stomach and pancreas. Interestingly, these three hormones regulate hunger, satiety and food intake.
=================================================
Google it
Ghrelin action in the brain controls adipocyte metabolism.
And there are far more many hormones involved in regulating hunger , satiety and body mass
Here is an intro of a good overview of the appetite regulation and you can google it “Hormonal Regulation of Food Intake”
———————————–
The brain regulates energy homeostasis in response
to signals from both adipose tissue and the
gastrointestinal tract. The drive to eat and energy expenditure
are adjusted so that over time, body weight
remains stable.
—————————————-
here is what they say about insulin, the so called “fat hormone”
—————————————-
There is considerable evidence that insulin acts as an
anorectic signal within the central nervous system (CNS).
Centrally administered insulin or an insulin mimetic decreases
food intake and body weight (8) and alters expression
of hypothalamic genes known to regulate food
intake. Insulin infusion into the third cerebral ventricle in
rodents (198) or lateral ventricle in primates (438) dosedependently
decreases food intake resulting in weight loss over a period of weeks. Intrahypothalamic (PVN)
insulin injection also decreases food intake and weight
gain in rats (275). Treatment with novel, orally available
insulin mimetics also decreases weight gain, adiposity,
and insulin resistance in mice on a high-fat diet (8). Conversely,
antibodies to insulin injected into the VMH of rats
stimulate food intake and repeated administration
of antiserum increases food intake and rate of weight gain
(270). Administration of antisense RNA against the insulin
receptor precursor protein results in hyperphagia and
increased fat mass (309). Similarly, neuron-specific deletion
of the insulin receptor results in obesity, hyperinsulinemia,
and dyslipidemia in male mice
—————————————-
================================================
So how does the brain regulate hunger, satiety and food intake? It doesn’t.
================================================
That is nonsense considering that all of these hormones have receptors in the brain and interact wider with the central nervous system and injecting them into the brain changes the behaviour artificially.
I see that you come from that anti insulin, low carb angle and you have much to learn if you really want to instead of projecting low carb anti insulin dogma that has absolutelly no basis in physiology and like all fad diets completelly excludes the one clear factor which is the brain.
@Vladex
I started with insulin and its role in adipocytes proliferation. I argued that insulin should cause the same effect systemically as it does locally. You disagreed by saying:
“The key word is “injecting” . When someone injects any hormone into the body , there will be an equal and oposite reaction. If you inject any hormone there will be opposite reactions to it.”
But now you present a paper on insulin and the CNS which supports my argument and opposes yours.
Tell you what, the idea that the brain controls all hormones? I concede it. You win this one. Congratulations.
Paul & Shou-Ching:
Great post! Great book! I’m waiting for the next installment on your obesity theory. That is a tough nut to crack. Keep up the good work. 🙂
Joe Mantle
Joe Mantle,
There will never be any real theory of obesity because quiet frankly we are not supposed to figure it out. There will be lots of hypothesis based on the various notions of fooling the body yet the body will always fight back against such concious actions.
It’s just like sex and relationships, it’s too important to be explained entirely by logic, civility or intellectualism.
@Paul
“Feedback to the brain about the quantity of fat mass comes to the brain through a hormone, leptin, that researchers can easily monitor; but feedback about the state of lean tissue comes through the nerves, which sense the state of tissues throughout the body.”
Have you considered pathology and how it fits your hypothesis?
Consider a C4 quadriplegic with the spinal cord severed and the only intact neuronal connections between brain and body limited to the vagus nerve (which is limited to visceral organs) and some limited autonomic and somatic innervation to the neck and trunk.
There will be intact autonomic circuits between the spinal cord and viscera and the somatic body, but these will be isolated from communication with the brain.
How do you propose the C4 quad regulates lean body mass via “nerves” in this situation? Are there unknown spinal ganglia in the myelon that do this?
I also have a problem with thinking of the hormonal system as “low bandwidth” with hundreds of peptides and hormones and almost infinite variability in receptor sensitivities and distribution and operating via the circulation at picogram quantities, while the nervous system is seen “high bandwidth”.
I think it is the reverse, in fact. When patients have visceral pain, the localization is so poor that we cannot even tell which organ or body region is hurting. A pleural effusion often causes pain in the ipsilateral shoulder 2 feet away. Spinal somatic nerves distribute to multiple areas and there is innervation of multiple areas by several nerves, etc.
I see the somatic and autonomic nervous system as hydraulic and the neurohormonal system with peptides and hormones as fly-by wire ; )
How do you imagine the nutritional status of lean tissue (which is of course many different types of tissue) for hundreds of nutrients is integrated and sent to the brain over afferent “nerves”?
How come the C4 quad does not suffer uncontrollable hypertrophy or wasting of the whole body due to interruption of your proposed feedback loop? (We know there is disuse atrophy but why does it stop?)
Hi Kurt,
Good questions … We discussed that earlier in the comment thread. By parallelism with what happens in amputated limbs and ghost pain, I would expect that there are “mental maps” of tissue quality. It is the mental maps that actually drive appetite; feedback from the nerves normally keeps the mental maps updated and accurate. As in the case of amputated limbs where “ghost pain” can occur, or tinnitus in the case of damaged aural nerves, the mental maps can sometimes become inaccurate when nerves are missing or damaged.
So I would expect that we’d see the brain lose sensitivity to the body in cases of widespread nerve damage, so that appetite becomes less correlated to the actual state of tissue. However, there would be more variance in appetite, rather than a fixed tendency for appetite to change in one particular direction, either hyperphagy or hypophagy. Some people might have hyper-appetite, others hypo-appetite, but most might remain close to normal.
Then there is the issue of whether changes in appetite would lead to changes in weight. Fat mass is not necessarily increased by increased appetite unless there is some additional pathology, creating the disease of obesity, which suppresses eg the leptin feedback loop. So a feedback loop leading to measurable weight fluctuations might operate only in the already-obese. Then the hyperphagy or hypophagy might lead to “ghost hyper-obesity” or “ghost hypo-obesity.”
Also, I am not talking about regulation of muscle mass, so I wouldn’t use the terms hypertrophy or wasting; though changes to food intake might affect the equilibrium muscle loss in our C4 quad.
So, in summary, my hypothesis works as an explanation of “The Fat Trap” but wouldn’t necessarily create specific observable consequences to body mass in the nerve damaged, but should increase the variance of appetite / food intake.
As far as who we might look to for a test case, I would guess that tissues of critical importance for life, like the heart and lungs, would matter the most in driving appetite. So I would bet that people in a vegetative state, who may need assistance feeding, breathing, or pumping blood, would be the ones who would show the greatest variance in appetite and therefore in weight. I have not looked for data on weight changes in such people.
You make good points about the nervous system. Of course, nerves and hormones are not distinct. The major neurotransmitters of the sympathetic nervous system, the catecholamines, are also systemic hormones.
I don’t know enough neurology to judge if this hypothesis actually works. I am putting it out there because I think it would explain some of the fact patterns seen in obesity, such as the fat trap.
Best, Paul
So how do you envision the nutritional status of tissues – deficiencies of micronutrients, etc. – being communicated via efferent nerve impulses?
And if the quadriplegics don’t waste away to nothing or overeat to massive obesity (the ones I know and observe directly do not do either), then what would be the function of a feedback loop that seems to be so unnecessary?
I think a better explanation for this is that a feedback loop via “nerves” that no neuroscientist has discovered yet, and that is not necessary to maintain lean mass probably does not exist.
Yes, epi and nepi are hormones by virtue of being elaborated in large enough amounts to have systemic effect by the adrenal medulla, which is a specialized sympathetic ganglion.
My point was that it seems to me somewhat easier to conceive of hormonal signaling than the much cruder neurogenic communication you propose.
I’d be very interested to see actual evidence of such if you uncover it.
It’s a hypothesis at this point, I’ll keep an eye out for evidence.
Hi Paul – great reading – both the book and the blog.
I am confused with rice – after following Paleo readings for a while, I was interested to read your thoughts on rice, but also on white rice over brown rice. When others talk about rice as being good for you, it is also brown rice over white rice (example at http://www.whfoods.com/genpage.php?tname=foodspice&dbid=128). Are you able to shed some light on this and help overcome my confusion.
Thanks!
Hi Caroline,
The “whole grain” movement I think is largely driven by (a) epidemiological studies showing that people who eat whole grains tend to be healthier than people who eat refined grains, (b) the presence of fiber which often appears to be beneficial in studies, (c) the presence of protein which might benefit vegetarians/vegans, and (d) lowered glycemic index compared to refined grains.
But the proteins are toxic, and grain fiber appears actually harmful in grain-specific studies — it looks like the beneficial fibers are from vegetables, fruits, and non-grain safe starches. Glycemic index doesn’t matter if you eat them as part of a meal. And epidemiological studies are distorted by two factors: (a) people who eat whole grains are health-conscious and do a lot of other healthy things, ie exercise, avoid smoking, avoid processed foods and excess alcohol, etc; and (b) a shift from refined grains to whole grains is generally also a shift away from wheat to other grains (ie “7 grain bread”) and other grains are healthier than wheat.
Overall, I think refined grains are healthier than whole grains on a low-carb diet in which the grains are eaten as part of a meal, combined with fat, healthy fiber, and acids. This is especially true of white rice over brown rice.
Hi Paul,
I am excited for your next installment and enjoyed this one quite a bit.
When it comes to the conversion of muscle tissue. I was reminded that Vitamin D can have a powerful impact on converting muscle to fast-twitch fibers.
http://www.charlespoliquin.com/ArticlesMultimedia/Articles/Article/708/Muscle_Size_Fast-Twitch_Fibers_and_Vitamin_D.aspx
I am excited to see how you’ll incorporate micronutrient status into muscle development in the prevention of yo-yoing. The effects of supplementing Vitamin D alone seem quite profound; could it significantly alter the course of extra slow-twitch caused by weight loss?
Thanks for your work and free blog posts 🙂
Rob
Hi Paul,
Awhile back via your blog you brought Paul Ewald to my attention (thank you), and after reading through some articles about him, I was struck by his frustration surrounding the lack of research into infection as a possible cause of dementia. He suggests that, after dementia in syphilitics was found to be caused by the bacterial infection, the majority of researchers went right back to assuming that dementia in non-syphilitic patients was not caused by an infection, rather than hypothesizing and investigating the opposite.
In your 2012.01.08 post you linked to an article by the Scientist re. parasites exerting mind control ( http://the-scientist.com/2012/01/01/animal-mind-control/ ). When I read it — shortly after reading your post here re. causes of obesity — a statement by the author jumped out at me: “Although researchers have observed countless examples of parasites hijacking the autonomy of their hosts, only now are they beginning to understand how the parasites tinker with numerous systems within the host, ultimately changing the host’s behavior in grotesque and horrific ways.”
The author seems to be making an assumption that: “Except for these few examples we’ve managed to discover thus far, the host’s behaviour is always autonomous.” This kind of statement sounds like a revision of a previously-held assumption — “The hosts behaviour is always autonomous” — which the examples in the article, and many more, have disconfirmed. But in the face of the evidence, it seems like it’s an assumption that shouldn’t go unchallenged.
It seems to me that another reasonable assumption/hypothesis to test is: “The hosts behaviour is never autonomous.” If there is evidence that certain passengers can contribute to aberrant behaviour in an animal, then it seems reasonable to hypothesize that, rather than normal behaviour indicating a lack of influence from passengers, it simply indicates influence from different passengers — ones that contribute to behaviour so widely observed that we call it normal.
Which brings me back to the New Scientist article, obesity, and feeding behaviour. In the article, “a normally insatiable caterpillar suddenly stops eating”, as a result of the wasp larvae in its body. I know you already believe that certain pathogens can affect some aspects of behaviour (i.e. C. Pneumoniae in the brain and its effects on cognition, mood, affect), but I’m wondering what your thoughts are re. pathogens — and/or gut flora (species and balance of species) — affecting feeding behaviour.
Thanks as always for your work, Paul. I can personally attest that it’s made a positive difference in at least three lives.
I meant to say, “Thanks as always for your and Shou Ching’s work”, Paul.
Hi Sammy,
Great points. I think it’s safe to say that human cognition is more autonomous than parasitic, since we’re the only species that has our kind of cognition. But parasites are pretty common. Much “odd” or “crazy” behavior could be parasite-influenced. The crazy lady with the cats is probably Toxoplasma gondii infected.
Yes, it’s certainly likely that gut flora and infections are a factor in obesity.
Thanks for letting me know about the 3 lives we’ve affected. We’d love to hear their stories on the Results page if they have time.
Best, Paul
@sammy,
There was a study that came out in 2010 about obesity and friends which was reported in many media outlets. The key quote: “We find that having four obese friends doubled people’s chance of becoming obese compared to people with no obese friends.”
I noticed at the time that the popular science press concluded it was due to the influence of “social networks”. (That’s the Internet period where everybody got really, really overexcited about social networks.) I wondered why nobody commenting on the study considered it could be caused by actual pathogens which manipulated human eating behavior, and these pathogens traveled between human hosts the same way that flu and colds make their way amongst friends.
I too have a bit of a problem with a lean tissue neurological feedback hypothesis. It was not that long ago that adipose tissue was considered biologically inactive. Then along came leptin, adiponectin, etc. Perhaps the chemical signalling mechanism of lean tissue has simply not yet been discovered?
Body builders routinely bulk up and then lose but appear to retain an extremely high RMR. It might serve us all well to add physiological studies to our nutritional ones.
In one of Stephan Guyenets’ food reward posts, he talks about the study where mice were fed different flavors of Ensure (meal replacement drink)and how the mice only overate and became obese on the chocolate flavor and not the vanilla or strawberry, which he suggests is evidence for the reward theory. The reason I bring this up is because I remember in a Robb Wolf podcast he said chocolate can interfere with iron absorption. I don’t how much of an effect it has or if there is enough chocolate in the Ensure, but if the chocolate flavored Ensure messed with iron absorption or other mineral/vitamin absorption (complete speculation) then it may be provoking the lean tissue response which would be responsible for the weight gain. Also I’m not so sure that chocolate is any more rewarding than vanilla or strawberry.
Hi James,
Great point. It should be possible to test this however – just give the mice a choice of chocolate, vanilla, and strawberry Ensure and see which they prefer. If they avoid vanilla and strawberry, it would show they find chocolate more rewarding. If they eat the flavors equally, it would show that overeating on the chocolate was due to a nutrient deficiency.
As part of the NWLR, and having maintained a weight loss of 50 pounds for 4-1/2 years, I have a couple of comments on long term effects.
My body still is defending the higher BMI fat level. I still have to eat 2200 calories a day to satisfy hunger. I compensate for this with activity, which has its own benefits. Maybe I was never meant to be sedentary in the first place. But adjusting one’s schedule to be this level of activivity is sometimes tricky, especially for a desk jockey.
At 0430 every day it’s not just ghrelin calling. If my stomach is empty I often feel empty in other ways. I’d suggest that you expand your theory to include psychological signaling. The last NWLR survey covered questions on feelings of self esteem, such as how regular you were in paying bills, and whether your job was stable. Consider all the other triggers for obesity. Metabolic setpoints aren’t the only thing in play, and may be minor compared to the psychological wells we fall into.