Mario Renato Iwakura is a Brazilian engineer and Hashimoto’s thyroiditis patient who is intimately familiar with the hypothyroidism literature. Mario has graciously agreed to do a guest series on the place of iodine and selenium supplementation in treatment of hypothyroid disorders. I’m very excited to have Mario’s thoughts, as he’s extremely smart and passionately engaged with the science. — Paul
Most doctors believe that iodine supplementation will aggravate autoimmune (Hashimoto’s) thyroiditis. This view is supported by observations that the incidence of Hashimoto’s hypothyroidism tends to increase in populations that increase their iodine intake. (The incidence of hyperthyroidism, on the other hand, increases as iodine intake decreases.). However not all epidemiological studies support this association [1][2][3][4].
Dr. Datis Kharrazian (“Dr. K”), whose 2010 book “Why Do I Still Have Thyroid Symptoms?”[5] is popular among Hashimoto’s patients, vehemently opposes the use of iodine in Hashimoto’s [5][6][7]. Chris Kresser of The Healthy Skeptic [8] has argued this point of view in his post “Iodine for hypothyroidism: like gasoline on a fire?”. And there’s little doubt that some patients have experienced bad consequences from high-dose iodine.
On the other side, doctors such as Dr. Guy E. Abraham [9], Dr. David Brownstein [10], Jorge D. Flechas [11] and Dr. David Derry [12] have claimed success prescribing high doses of iodine for Hashimoto’s and for breast and thyroid cancers.
Can these experiences by reconciled? What we will try to do is demonstrate that iodine acts synergistically with selenium, and that it is imbalances between the two that damage the thyroid.
First, Some Background
Thyroid peroxidase or thyroperoxidase (TPO) is an enzyme expressed mainly in the thyroid that liberates iodine for addition onto tyrosine residues on thyroglobulin (TG) for the production of the thyroid hormones thyroxine (T4) or triiodothyronine (T3).
The human body normally has low levels of auto-antibodies against both TG and TPO, which serve some physiological function. Autoimmune thyroiditis features high levels of these auto-antibodies, leading to immune attacks on the thyroid.
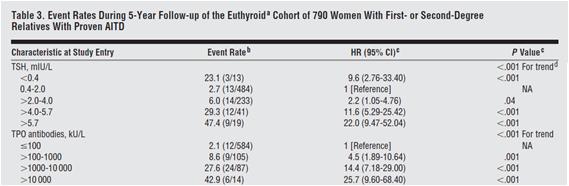
High levels of thyroid auto-antibodies are positively associated with hypothyroidism symptoms [13][14]. TPO antibodies and TSH levels are strongly associated with progression of subclinical hypothyroidism to overt hypothyroidism [3], as can be see in Table 3 below:
Selenium Can Cure An Iodine Excess
Dr. K said in his book and site that “iodine stimulates the production and activity of the thyroid peroxidase (TPO) enzyme” [5][7]. Since TPO is a target of autoimmune attack in Hashimoto’s patients, this might worsen the disease [5][6][7]. In his book he also states that excessive iodine will shut down TPO activity [5], but he neither cites a reference nor states what level of iodine intake will cause this to happen.
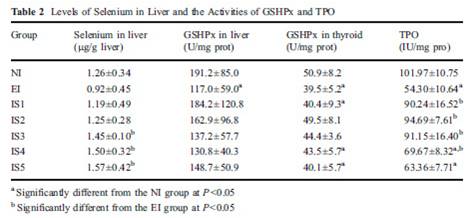
In fact, excess iodine combined with selenium insufficiency will reduce (not increase, not shut down) TPO activity [15]. Let’s look at a study that had seven groups: normal iodine and lab-chow selenium only (NI), excess iodine and lab-chow selenium only (EI), and five groups with excess iodine and steadily increasing levels of selenium added to water (IS1 to IS5). TPO activity was reduced by excess iodine (EI), but returned to control levels (NI) with moderate selenium (IS1 and IS2). With excess iodine and excessive selenium (IS3 to IS5), TPO activity was also decreased, as we can see from table 2 below.
Some other studies have also demonstrated this reduced TPO activity at high iodine intakes [23][24].
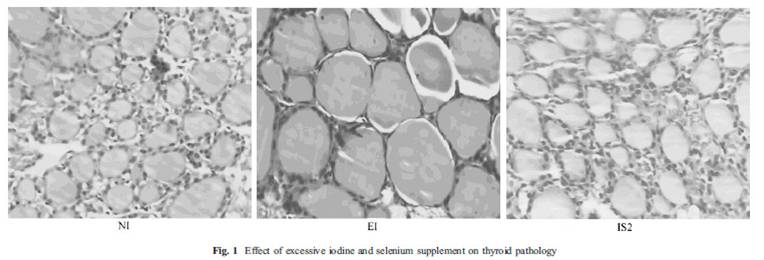
This study [15] also showed a picture (fig. 1) of thyroid follicles from rats receiving normal iodine diet (NI), excessive iodine (EI) and excessive iodine plus 0.2 mg/L selenium (IS2). Thyroid follicles from the excessive iodine group (EI) are enlarged, a characteristic of goiter. But, there is virtually no difference between the first and last picture! If selenium and iodine are increased together, no goiter occurred.
Note that the IS2 level of selenium, which protects against iodine toxicity, corresponds in a person who drinks 1-2 liters per day to a selenium dose of 200 to 400 mcg per day – which happens to be the Perfect Health Diet “plateau range” for selenium.
Selenium Can Cure Autoimmunity
Another paper, also from China, looked at the effects of selenium in an animal model of iodine induced autoimmune thyroiditis [16].
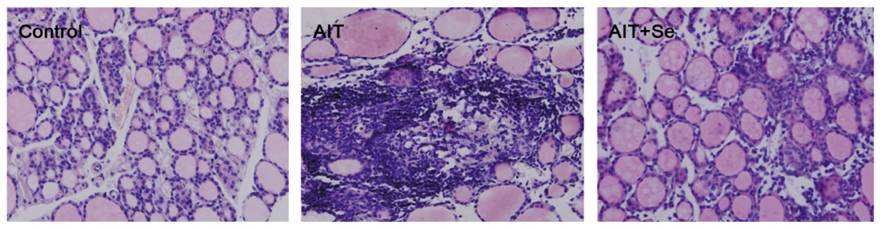
There were three groups of mice, a healthy control group, and groups with iodine induced autoimmune thyroiditis without (AIT) and with (AIT+Se) selenium. The AIT+Se group was given high iodine (AIT only) for 8 weeks to induce the disease, and then, for 8 weeks more, they were given iodine plus selenium. After 8 weeks of selenium supplementation their thyroid follicles were almost fully recovered, as we can see below, even though high-dose iodine had continued:
The AIT group has enlarged cells characteristic of goiter and dead tissue; the AIT-Se group thyroid section resembles a normal thyroid. Thyroid weight doubled in the AIT group, proof of goiter, but returned to normal after selenium supplementation.
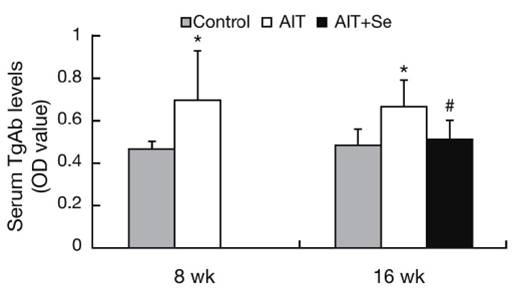
Before selenium was given to the AIT+Se group, serum TgAb antibodies were elevated, but they returned to normal after selenium supplementation:
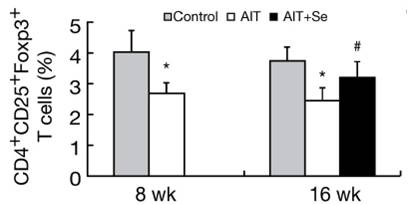
An interesting aspect of this study was the changing population of immune cells. A specialized subpopulation of T cells, negative regulatory T cells or Tregs, helps establish and maintain self-tolerance by suppressing response to self-antigens and suppressing excessive immune responses deleterious to the host. Deficits in Treg cell numbers or function lead to autoimmune diseases [17].
In this study, CD4+CD25+Foxp3+ Treg Cells were reduced by high iodine, but returned much of the way toward normal after 8 weeks of selenium even though high iodine intake continued. The implication is that selenium-iodine balance may be needed to maintain proper Treg cell populations, and that selenium supplementation may restore normal regulation of autoimmunity.
The researchers concluded:
“In the present study, we observed that Se supplementation increased the frequency of CD4+CD25+Foxp3+ T cells and enhanced expression of Foxp3 in vivo. These changes were accompanied by suppressed TgAb titers and reduced thyroiditis. Thus the benefit of Se treatment may be due to the increase of CD4+CD25+ regulatory T cells.”
Under What Circumstances Does Excess Iodine Induce Autoimmunity?
In the previous study high doses of iodine were used to induce autoimmune thyroiditis. Let’s look more closely into the circumstances in which that happens.
It’s often said that excessive iodine in Hashimoto’s triggers an immune response characterized by proliferation of T lymphocytes, a disrupted Th1/Th2 axis, and altered CD4/CD8 levels. Pathogenesis of autoimmune disease is believed to begin with the activation of T cell autoaggression (turning them into “allergized T cells”).
Our next study, also from China, showed that excess iodine can indeed cause such an autoimmune pathology, but only if there is a deficiency in selenium [18].
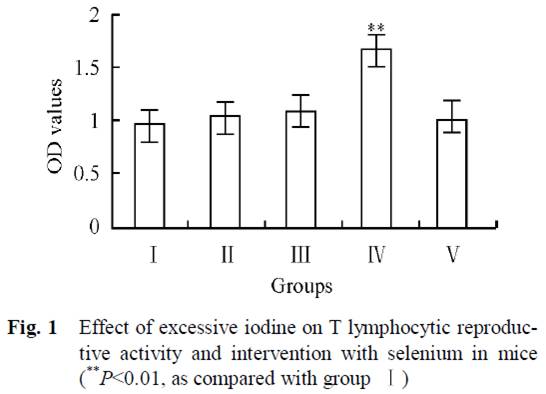
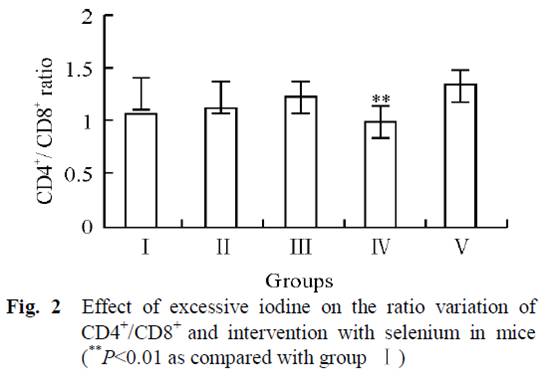
Mice in 5 groups were orally administrated different combinations of iodine and selenium for 30 days. Four groups had no selenium but varying amounts of iodine in their water: 0 μg/L (group I), 1500 μg/L (group II), 3000 μg/L (group III), and 6000 μg/L (group IV). The fifth group had 6000 μg/L iodine plus 0.3 mg/L selenium (group V).
In Group IV, high-dose iodine at 6000 μg/L caused a proliferation of lymphocytes. But this was completely abolished by the addition of selenium to water in Group V:
Normally there are relatively stable population of T cells and their subgroups in tissue till immune function is in disorder. As we can see from Fig. 1, increasing iodine increased T lymphocytic reproductive activity, and was clearly high in group IV. But group V, which also received selenium, had the same values as the control group (I).
Subjects with Hashimoto’s also have a lower ratio of CD4+ to CD8+ lymphocytes than controls [19][20]. From fig. 2, we can see that iodine supplementation in groups II and III actually increased the CD4+ to CD8+ ratio, until the onset of autoimmune symptoms at very high doses in Group IV when the ratio decreased. However, group V, which had the highest iodine intake but with selenium as well, had the highest CD4+ to CD8+ ratio of all groups. This suggests that high-dose iodine and selenium together may actually diminish the autoimmune syndrome compared to the low levels in the controls.
Another marker of autoimmune thyroiditis is the relative strength of the Th1 and Th2 responses, as indicated by the markers interferon-gamma and interleukin-4 (Th2). Th1(IFN-γ)/Th2(IL-4) ratios are increased in Hashimoto patients [21][22], and related with severity of Hashimoto’s disease [22].
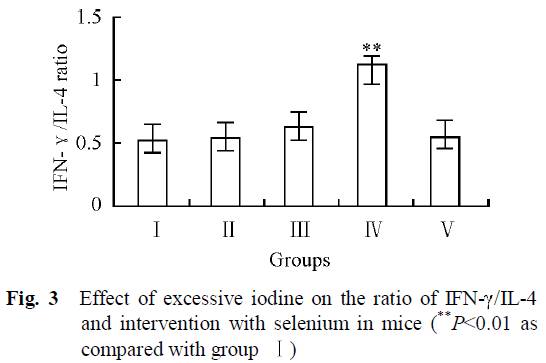 As we can see from Fig. 3, the group with the highest iodine intake but no selenium (IV) was the only group that had clearly higher Th1/Th2 ratio. High iodine plus selenium in group V had similar Th1/Th2 ratios than control group (I).
As we can see from Fig. 3, the group with the highest iodine intake but no selenium (IV) was the only group that had clearly higher Th1/Th2 ratio. High iodine plus selenium in group V had similar Th1/Th2 ratios than control group (I).
The researchers concluded:
“The results revealed that there was no significant difference in the immunotoxicity between interventional group (group V) and control group (group I), indicating that adequate selenium has a favorable interventional effect on excessive iodine intake.”
Conclusion
Excess iodine intake can cause an autoimmune thyroiditis that bears all the characteristics of Hashimoto’s. However, in animal studies this occurs only if selenium is deficient or in excess. Similarly, in animal studies very high iodine intake can exacerbate a pre-existing autoimmune thyroiditis, but only if selenium is deficient or in excess.
With optimal selenium status, thyroid follicles are healthy, goiter is eliminated, and autoimmune markers like Th1/Th2 ratio and CD4+/CD8+ ratio are normalized over a wide range of iodine intake. It seems that optimizing selenium intake provides powerful protection against autoimmune thyroid disease, and provides tolerance of a wide range of iodine intakes.
In the next post in this series (Iodine and Hashimoto’s Thyroiditis, Part 2, May 26, 2011), we’ll transition from animals to humans. Does epidemiological evidence suggest that these animal findings are transferable to humans?
References:
[1] F. Aghini-Lombardi et al. The spectrum of thyroid disorders in an iodine-deficient community: the Pescopagano Survey. J. Clin. Endocrinol. Metab. 84, 561–566 (1999). http://pmid.us/10022416.
[2] Marino MA et al. Urinary iodine in patients with auto-immune thyroid disorders in Santo André, SP, is comparable to normal controls and has been steady for the last 10 years. Arq Bras Endocrinol Metabol. 2009 Feb;53(1):55-63. http://pmid.us/19347186.
[3] Strieder TG et al. Prediction of progression to overt hypothyroidism or hyperthyroidism in female relatives of patients with autoimmune thyroid disease using the Thyroid Events Amsterdam (THEA) score. Arch Intern Med. 2008 Aug 11;168(15):1657-63. http://pmid.us/18695079.
[4] Stuckey BG et al. Low urinary iodine postpartum is associated with hypothyroid postpartum thyroid dysfunction and predicts long-term hypothyroidism. Clin Endocrinol (Oxf). 2011 May;74(5):631-5. doi: 10.1111/j.1365-2265.2011.03978.x. http://pmid.us/21470286.
[5] Dr. Datis Kharrazian. Why Do I Still Have Thyroid Symptoms? When My Lab Tests Are Normal: A Revolutionary Breakthrough In Understanding Hashimoto’s Disease and Hypothyroidism.
[6] Dr. Datis Kharrazian. Iodine and Autoimmune Thyroid — References. http://drknews.com/some-studies-on-iodine-and-autoimmune-thyroid-disease/.
[7] Dr. Datis Kharrazian. Iodine and Hashimoto’s. http://drknews.com/iodine-and-hashimotos/.
[8] Chris Kresser. Iodine for hypothyroidism: like gasoline on a fire?. http://thehealthyskeptic.org/iodine-for-hypothyroidism-like-gasoline-on-a-fire.
[9] Dr. Guy E. Abraham. http://www.optimox.com/.
[10] Dr. Brownstein. Iodine, Why You Need It. https://www.drbrownstein.com/homePage.php.
[11] Dr. Jorge D. Flechas. http://cypress.he.net/~bigmacnc/drflechas/index.htm.
[12] Dr. David Derry. Breast Cancer and Iodine : How to Prevent and How to Survive Breast Cancer.
[13] Ott J et al. Hashimoto’s thyroiditis affects symptom load and quality of life unrelated to hypothyroidism: a prospective case-control study in women undergoing thyroidectomy for benign goiter. Thyroid. 2011 Feb;21(2):161-7. Epub 2010 Dec 27. http://pmid.us/21186954.
[14] Díez JJ, Iglesias P. Relationship between thyrotropin and body mass index in euthyroid subjects. Exp Clin Endocrinol Diabetes. 2011 Mar;119(3):144-50. Epub 2010 Nov 17. http://pmid.us/21086247.
[15] Xu J et al. Supplemental Selenium Alleviates the Toxic Effects of Excessive Iodine on Thyroid. Biol Trace Elem Res. 2010 Jun 2. http://pmid.us/20517655.
[16] Xue H et al. Selenium upregulates CD4(+)CD25(+) regulatory T cells in iodine-induced autoimmune thyroiditis model of NOD.H-2(h4) mice. Endocr J. 2010 Jul 30;57(7):595-601. Epub 2010 Apr 27. http://pmid.us/20453397.
[17] Sakaguchi S et al. Foxp3+CD25+CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006 Aug;212:8-27. http://pmid.us/16903903.
[18] Chen X et al. Effect of excessive iodine on immune function of lymphocytes and intervention with selenium. J Huazhong Univ Sci Technolog Med Sci. 2007 Aug;27(4):422-5. http://pmid.us/17828501.
[19] Gopalakrishnan S et al. The role of T-lymphocyte subsets and interleukin-5 blood levels among Indian subjects with autoimmune thyroid disease. Hormones (Athens). 2010 Jan-Mar;9(1):76-81. http://pmid.us/20363725.
[20] Zeppa P et al. Flow cytometry phenotypization of thyroidal lymphoid infiltrate and functional status in Hashimoto’s thyroiditis. Anal Quant Cytol Histol. 2006 Jun;28(3):148-56. http://pmid.us/16786724.
[21] Colin IM et al. Functional lymphocyte subset assessment of the Th1/Th2 profile in patients with autoimmune thyroiditis by flowcytometric analysis of peripheral lymphocytes. J Biol Regul Homeost Agents. 2004 Jan-Mar;18(1):72-6. http://pmid.us/15323363.
[22] Nanba T et al. Increases of the Th1/Th2 cell ratio in severe Hashimoto’s disease and in the proportion of Th17 cells in intractable Graves’ disease. Thyroid. 2009 May;19(5):495-501. http://pmid.us/19415997.
[23] Müller K et al. Effect of iodine on early stage thyroid autonomy. Genomics. 2011 Feb;97(2):94-100. http://pmid.us/21035537.
[24] Man N et al. Long-term effects of high iodine intake: inhibition of thyroid iodine uptake and organification in Wistar rats. Zhonghua Yi Xue Za Zhi. 2006 Dec 26;86(48):3420-4. http://pmid.us/17313856.











Holy cow this is an amazing analysis! Looking foreward to reading your next article! Thanks for your work!