A number of people asked for comments on the most recent red meat scare, including Nicole, Ryan, and Mishkin on the blog, JT Olds on Twitter, and others on Facebook. You probably saw some of the headlines:
- “Why eating red meat raises cancer risk” (San Diego Union-Tribune)
- “Scientists crack why red meat is linked with cancer – and SUGAR may be to blame” (Daily Mail)
- “Humans not built to eat cancer-causing red meat” (Examiner.com)
- “Red meat triggers toxic immune reaction which causes cancer, scientists find” (UK Telegraph)
The article Nicole linked is a bit more scientifically inclined: “Possible Link Between Red Meat Consumption And Increased Cancer Risk Identified” (IFL Science). Here’s the press release version from UCSD: “Sugar Molecule Links Red Meat Consumption and Elevated Cancer Risk in Mice”. In the blogosphere, Stephan has summarized the issue in the context of a post on red meat and cancer.
The headlines are based on a paper [01] that reported that, in mice genetically altered to lack a sugar (Neu5gc) that humans also lack, feeding Neu5gc and injecting anti-Neu5gc antibodies generates inflammation which can promote the growth of cancers.
Significance of Neu5gc
The paper itself is a rather artificial scenario whose significance will be determined by future work. So analyzing this single paper would not be interesting. But I think it’s worthwhile to look into the broader idea that eating Neu5gc-bearing meats might be inflammatory or a source of autoimmunity.
In terms of PHD recommendations, this could affect the relative emphasis we place on different meats. If Neu5Gc is a true health risk, then we would want to emphasize seafood more and red meat less.
Another benefit to thinking about Neu5gc is that it may give us some insight into what a PHD “autoimmune protocol” should look like.
Background: Evolutionary History of Neu5gc
All cells in multicellular organisms are coated in carbohydrates, and the carbohydrates terminate in one of 43 sialic acids. In mammals, two forms of predominate: Neu5Ac and Neu5Gc. Each mammalian cell has tens or hundreds of millions of molecules of Neu5Ac and Neu5Gc on its surface. [02]
Neu5Gc is made from Neu5Ac, but the gene for making Neu5Gc was inactivated in the human lineage shortly before the emergence of Homo. The mutation occurred 3.2 million years ago and reached fixation – that is, all ancestral hominids had come to have the mutated gene – 2.9 million years ago. [03] This very rapid fixation indicates there was strong selection in support of the mutation.
In fact, this mutation by itself may have led to a speciation event, after which our ancestors could no longer mate with other apes. From that point on, Neu5gc-less females had difficulty producing children with males who retained the Neu5gc gene, because they would form antibodies against Neu5gc-coated sperm, making fertilization unlikely. [04]
Why was losing Neu5gc selected in our ancestors? Two possibilities are likely:
- Loss of Neu5gc improved brain function.
- Loss of Neu5gc (temporarily) reduced vulnerability to (ancestral) pathogens.
It should be noted that Neu5Gc has been lost independently in some other mammals as well – ferrets and new world monkeys. New world monkeys such as capuchins and spider monkeys also experienced a brain expansion, and ferrets are notably smart, so either explanation might be relevant to these cases of “convergent evolution.”
Neu5gc and Brain Function
Carbohydrates are extremely important for intercellular interactions. Indeed, the incorporation of carbohydrates into cell membranes and extracellular matrix is what made possible the rise of multicellular life.
In no organ are intercellular interactions as complex or consequential as in the brain. Not surprisingly, then, carbohydrates including the sialic acids are important to brain function.
The human brain is extraordinarly rich in sialic acids: neural membranes have 20 times more sialic acids than membranes of other human cell types. Animal brains are also enriched in sialic acids relative to their other tissues, but not as much as in humans; the human brain has 2-4 times more sialic acids the brains of other mammals. [05]
Curiously, though, Neu5gc is rare in the brains of all animals. Neu5gc is strongly suppressed, by about 98%, in the brains of all vertebrates, suggesting that its presence inhibits brain function. [06] It appears that Neu5gc is somehow toxic to brain function.
Loss of the gene for Neu5gc completely eliminated Neu5gc from the hominid brain. If Neu5gc does impair brain function, mutational inactivation of Neu5gc would have improved brain function. If so, the mutational inactivation of Neu5gc could have been driven by the same evolutionary forces that, soon after, selected for the tremendous expansion of the hominid brain.
Incidentally, dietary sialic acids — except for Neu5Gc – appear to be nutritious for humans, and especially for the developing infant brain. Breast milk is exceptionally rich in sialic acids, almost all of it Neu5Ac. Formula, by contrast, has much lower levels of sialic acids (0.21 mmol/L compared to 3.72 mmol/L in breast milk). Breast fed infants have nearly twice as many sialic acids in saliva than formula fed infants, confirming that milk sialic acids are taken up by the body and utilized.
Animal studies show that sialic acids in breast milk nourish the brain. Sialic acids facilitate neurotransmission between neurons. When piglet milk is supplemented with sialic acids, brain sialic acid levels are increased, and the piglets learn faster and make fewer mistakes in maze tests. [05] Rodents also perform better on tests of learning and memory after sialic acid supplementation. [07]
Not only does formula have fewer sialic acids than breast milk, cow milk based formulas have some Neu5Gc. [05] It has been observed that formula-fed infants have lower IQs than breast-fed infants. Sialic acids might help explain that. The lack of nourishing Neu5Ac and the presence of toxic Neu5Gc in formula might lastingly impair brain function in formula-fed infants.
Neu5Gc and Infection Risk
As the outermost molecules in the carbohydrate coat surrounding cells, sialic acids are the first contact point for pathogens seeking entry to the cell, and for immune cells seeking to detect whether the cell is native or foreign.
There has been a “Red Queen” evolutionary arms race in which pathogens evolved ways to utilize sialic acids for cell entry, or to hide from the immune system; and animals evolved changes to their sialic acids to frustrate the pathogens. [08]
Many pathogens interact with sialic acids in order to adhere to and gain entry into the cell. Pathogens generally rely on a single specific endocytic route for cell entry. This often requires binding to a specific sialic acid as the initial point of attachment.
Pathogens that specifically utilize Neu5Gc to enter cells include canine and feline parvoviruses [09]; pathogens that specifically utilize Neu5Ac include adeno-associated viruses and the minute virus of mice (MVM) [10].
A human pathogen that uses sialic acids to enter cells is the malaria protozoan. Plasmodium falciparum causes severe disease in humans and enters cells via Neu5Ac; Plasmodium reichenowi causes milder disease in chimpanzees and gorillas and enters cells via Neu5Gc. Plasmodium falciparum appears to have evolved recently – possibly reaching its current form only 10,000 years ago when the rise of agriculture and animal husbandry brought humans and mosquitos into closer proximity – while Plasmodium reichenowi is thought to resemble the ancestral form that would have afflicted hominids and apes 3.5 million years ago.
Possibly, the gene for Neu5Gc was inactivated to protect ancestral hominids from malaria. With the loss of Neu5Gc, hominids would have become immune to P. reichenowi. [11] [12]
Unfortunately, after P. falciparum’s adaptation to Neu5Ac which is overabundant in humans, we now suffer from more severe malaria than chimpanzees or gorillas (the “malignant malaria” mystery). [13]
In addition to entry points for microbes, sialic acids can be entry points for microbial toxins. For example, Shiga toxin from shigatoxigenic E. coli binds to Neu5Gc. [14]
Sialic Acid Concealment and the Gut Microbiome
The immune system is sensitive to the composition of the carbohydrate coat on a cell. White blood cells have a number of sialic acid detectors on their surfaces (called Siglecs, for sialic acid Ig-superfamily lectins). Some, which bind to human sialic acids, inhibit immune responses. Others, which bind to non-human sialic acids, activate immune responses.
Thus, when white blood cells contact a cell bearing human sialic acids, the immune system interprets it as “self” and tamps down immunity. When it detects foreign sialic acids, the immune system treats the cell as “foreign” and is more likely to attack it.
Some microbes – including commensal gut microbes – have been living in humans long enough that they have learned to take up sialic acids, chiefly Neu5Ac, and incorporate them into lipopolysaccharides on their cell membranes. This suppresses immunity toward them. [15]
A number of human pathogens have learned the same trick. Pathogens that incorporate sialic Neu5Ac into their cell membranes for the purpose of mimicking human cells and evading human immune defenses include Escherichia coli K1, Haemophilus influenzae, Pasteurella multocida, Neisseria spp., Campylobacter jejuni and Streptococcus agalactiae. [16]
Due to this “molecular mimickry” of human molecules, it has been suggested that these bacteria – especially Haemophilus influenzae and Neisseria spp. – may be sources of autoimmunity. [17]
While some bacteria can synthesize sialic acids themselves, most obtain it from their environment. These bacteria release enzymes called sialidases to cleave the sialic acids from food in the digestive tract, from surrounding cells, or from mucus. [15] Bacteria can obtain Neu5Ac from human tissue and mucus as well as food, but Neu5Gc only from food, chiefly beef and pork.
Neu5Gc in Human Tissue
Although humans can no longer synthesize Neu5Gc, we still have all the cellular machinery for utilizing it. When dietary Neu5Gc is absorbed into the body and enters cells, it can be incorporated into glycoproteins bound for the cell surface glycocalyx, just as Neu5Ac is.
As a result, Neu5Gc of dietary origin appears at low levels on the surface of human cells.
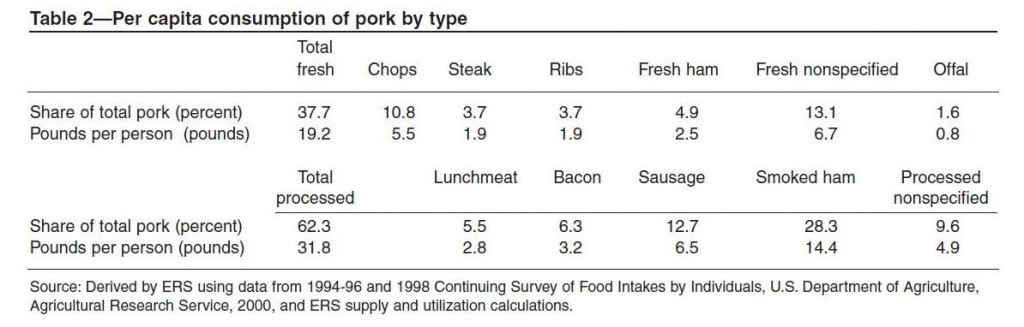
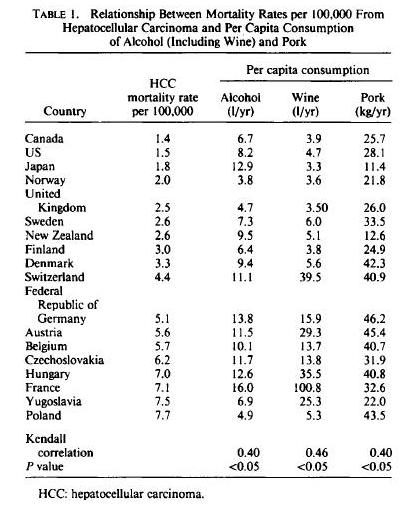
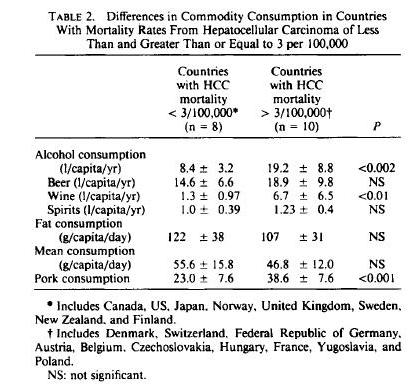
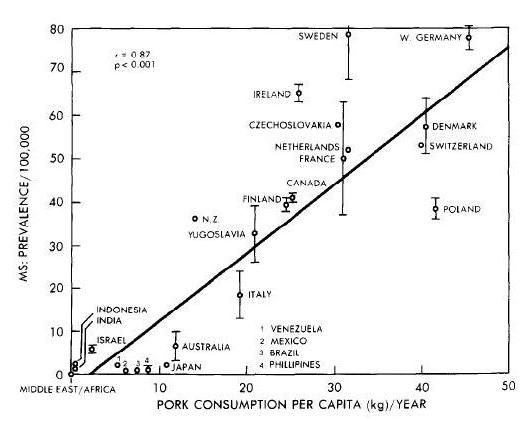
Neu5gc is found at high levels in all mammals except humans, ferrets, and new world monkeys; birds and reptiles do not produce Neu5Gc at all, and fish and shellfish produce only low levels. So, of the four major meat groups – beef, pork, chicken, and fish – Neu5gc is obtained predominantly from the red meats, beef and pork.
Among human cells, Neu5Gc appears at highest levels on tumor cells, especially metastatic cells. [21] This makes Neu5Gc a potential target for cancer therapy.
Neu5Gc as an Immunogen
Neu5gc expressed on cell walls is a potential immunogen. When pig organs are transplanted to humans, Neu5Gc is the second most important cause of rejection, after the α1,3-galactose (αGal) epitope. [20]
Anti-Neu5gc antibodies have been found in 85% of humans. [18] It is thought that antibodies form in early childhood after dietary Neu5Gc is incorporated by certain gut bacteria into lipooligosaccharides that can generate antibodies. Some of these antibodies may cross-react with compounds human cells form from dietary Neu5Gc; these human molecules are then known as “xeno-autoantigens.” [21]
Summary
Neu5Gc from mammalian meats, such as beef and pork, is incorporated into the cell surface coats and walls of gut microbes and some human cells, mainly in the gut and in tumors. Neu5gc in bacterial walls is immunogenic and 85% of people have detectable antibodies to Neu5Gc. Eating beef and pork supplies antigens for these antibodies, potentially triggering inflammation. There are concerns that this inflammation may have negative health effects.
Next up: Neu5Gc and autoimmunity.
Note: May Perfect Health Retreat
Interested in a luxury vacation combined with an education in how to be healthy for the rest of your life? Science classes, cooking classes, movement and relaxation classes, personal health coaching, gourmet food, all on a magnificent beach, with hot tubs and salt water pools? Come to the Perfect Health Retreat! Spaces are currently 2/3 filled and the retreat is expected to sell out early. Visit here for more info or email me at paul@perfecthealthretreat.com.
References
[01] Samraj AN et al. A red meat-derived glycan promotes inflammation and cancer progression. Proc Natl Acad Sci U S A. 2014 Dec 29. pii: 201417508. [Epub ahead of print]. http://pmid.us/25548184.
[02] Kraemer PM. Sialic acid of mammalian cell lines. J Cell Physiol. 1966 Feb;67(1):23-34. http://pmid.us/5327858. Was 21
[03] Hayakawa T, Aki I, Varki A, Satta Y, Takahata N. Fixation of the human-specific CMP-N-acetylneuraminic acid hydroxylase pseudogene and implications of haplotype diversity for human evolution. Genetics. 2006 Feb;172(2):1139-46. http://pmid.us/16272417. Full text: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1456212/. Was 22
[04] Ghaderi D et al. Sexual selection by female immunity against paternal antigens can fix loss of function alleles. Proc Natl Acad Sci U S A. 2011 Oct 25;108(43):17743-8. http://pmid.us/21987817. was 2
[05] Wang B. Molecular mechanism underlying sialic acid as an essential nutrient for brain development and cognition. Adv Nutr. 2012 May 1;3(3):465S-72S. http://pmid.us/22585926. Full text: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3649484/. Was 31
[06] Davies LR, Varki A. Why Is N-Glycolylneuraminic Acid Rare in the Vertebrate Brain? Top Curr Chem. 2013 Mar 8. [Epub ahead of print] http://pmid.us/23471785. was 8
[07] Wang B. Sialic acid is an essential nutrient for brain development and cognition. Annu Rev Nutr. 2009;29:177-222. http://pmid.us/19575597. was 32
[08] Varki A. Colloquium paper: uniquely human evolution of sialic acid genetics and biology. Proc Natl Acad Sci U S A. 2010 May 11;107 Suppl 2:8939-46. http://pmid.us/20445087. was 51
[09] Löfling J et al. Canine and feline parvoviruses preferentially recognize the non-human cell surface sialic acid N-glycolylneuraminic acid. Virology. 2013 May 25;440(1):89-96. http://pmid.us/23497940. was 54
[10] Wu Z et al. Alpha2,3 and alpha2,6 N-linked sialic acids facilitate efficient binding and transduction by adeno-associated virus types 1 and 6. J Virol. 2006 Sep;80(18):9093-103. http://pmid.us/16940521. was 55
[11] Varki A, Gagneux P. Human-specific evolution of sialic acid targets: explaining the malignant malaria mystery? Proc Natl Acad Sci U S A. 2009 Sep 1;106(35):14739-40. http://pmid.us/19717444. was 57
[12] Martin MJ et al. Evolution of human-chimpanzee differences in malaria susceptibility: relationship to human genetic loss of N-glycolylneuraminic acid. Proc Natl Acad Sci U S A. 2005 Sep 6;102(36):12819-24. http://pmid.us/16126901. was 58
[13] Rich SM et al. The origin of malignant malaria. Proc Natl Acad Sci U S A. 2009 Sep 1;106(35):14902-7. http://pmid.us/19666593/.
[14] Byres E et al. Incorporation of a non-human glycan mediates human susceptibility to a bacterial toxin. Nature. 2008 Dec 4;456(7222):648-52. http://pmid.us/18971931.
[15] Varki A, Gagneux P. Multifarious roles of sialic acids in immunity. Ann N Y Acad Sci. 2012 Apr;1253:16-36. http://pmid.us/22524423. Full text: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3357316/
[16] Severi E, Hood DW, Thomas GH. Sialic acid utilization by bacterial pathogens. Microbiology. 2007 Sep;153(Pt 9):2817-22. http://pmid.us/17768226. Full text: http://mic.sgmjournals.org/content/153/9/2817.long.
[17] Harvey HA, Swords WE, Apicella MA. The mimicry of human glycolipids and glycosphingolipids by the lipooligosaccharides of pathogenic neisseria and haemophilus. J Autoimmun. 2001 May;16(3):257-62. http://pmid.us/11334490.
[18] Zhu A, Hurst R. Anti-N-glycolylneuraminic acid antibodies identified in healthy human serum. Xenotransplantation. 2002 Nov;9(6):376-81. http://pmid.us/12371933.
[19] Takahashi T et al. N-glycolylneuraminic acid on human epithelial cells prevents entry of influenza A viruses that possess N-glycolylneuraminic acid binding ability. J Virol. 2014 Aug;88(15):8445-56. http://pmid.us/24829344.
[20] Park JY et al. α1,3-galactosyltransferase deficiency in germ-free miniature pigs increases N-glycolylneuraminic acids as the xenoantigenic determinant in pig-human xenotransplantation. Cell Reprogram. 2012 Aug;14(4):353-63. http://pmid.us/22775484.
[21] Samraj AN, Läubli H, Varki N, Varki A. Involvement of a non-human sialic Acid in human cancer. Front Oncol. 2014 Feb 19;4:33. http://pmid.us/24600589.




















Recent Comments