A few “Peat-atarians” – followers of the iconoclastic health writer Ray Peat – have accused us of being too skeptical of fructose. They think we should promote sugar consumption.
Here’s Travis Culp:
I think fructose is only conditionally problematic and that the consumption of it alongside glucose at a time of low liver glycogen is highly advantageous. In fact, I would go so far as to say that (somewhat slowly) drinking a can of soda upon waking (as disgusting as that is) would not result in any real glycation, insulin resistance, elevated TGs etc…. I think it’s beneficial to eat something really sugary upon waking …
Here’s Danny Roddy:
Peat has stated that … fructose … “powerfully” refills glycogen …
I would consider the ability to refill glycogen (minimizing adrenaline & cortisol release) to be an important factor in health …
It’s true that the ability to refill glycogen is essential for health; some genetic glycogen storage disorders are fatal in early childhood. But everyone who lacks a glycogen storage disorder has the ability to refill glycogen from multiple sources. In addition to fructose, glucose sugars and starches refill glycogen, as does milk sugar (a compound of glucose and galactose).
So the question is which combination of dietary sugars (a) is best at refilling glycogen and (b) makes the healthiest diet, all things considered?
Sugar Composition of the Diets
Both Danny and Travis framed their arguments as criticisms of our diet. They are really arguing that a Peat-style sugary diet is healthier than a PHD-style moderate-starch diet.
So before going further, let’s look at the sugar content of the diets we’re comparing.
First, note that PHD is not a zero-fructose diet. As an examination of the PHD Food Plate shows, PHD includes many fructose-containing plant foods – fruits, berries, and vegetables such as beets, onions, carrots, and squashes – plus “pleasure foods” like chocolate.
Also, PHD is not a zero-dairy diet, so for many practitioners it will include some milk sugars which are half galactose and half glucose.
In my diet personally, probably about 55% of carb calories come from starches, 30% from fruits, berries, and sugary vegetables, and 15% from dairy products such as yogurt. In terms of simple sugars, this translates to about 77% glucose, 15% fructose, and 8% galactose.
Not every Perfect Health Dieter will have the same sugar proportions; there is no obligation to consume dairy, and the relative proportions of starchy and sugary plants will vary according to taste. But let’s take mine as characteristic PHD proportions.
In a Peat-style diet, in contrast, the breakdown of sugars is near 50% glucose and 50% fructose.
So we aren’t comparing fructose against glucose, but a 77% glucose 15% fructose diet against a 50% glucose 50% fructose diet.
Why the Focus on Refilling Glycogen?
Why do the defenders of sugar focus on its ability to refill glycogen?
The reason is that fructose is treated by the body as a poison. Dietary fructose is shunted to the liver for disposal by conversion to glycogen, fat, lactate, or pyruvate.
Fructose is treated like a poison because it is dangerous. High doses of fructose have observable harmful effects even in short-term studies. Fructose does no good to the liver while it’s there, in fact fructose combined with polyunsaturated fats very effectively creates liver disease. Fructose in any other organ does harm; for instance, fructose promotes cancer growth.
Given fructose’s rapid disposal, any benefits from fructose have to be attributable to the glycogen or other products it is turned into. If fat, lactate, or pyruvate (a glucose product) provided benefits, dietary fats or starches would do the same, without the risk of fructose toxicity or fats getting stuck in the liver due to choline and methionine deficiency. So if fructose is to have benefits, it has to be via glycogen.
Here, then, is the challenge Peat-atarians face. Fructose has many proven harms. It has only one possible benefit: its ability to help re-fill liver glycogen. Peat-atarians have to show two things:
- That a diet with Peat-like sugar proportions – roughly 50% fructose, 50% glucose –is better than a diet with PHD-like sugar proportions – 15% fructose, 8% galactose, 77% glucose – at refilling liver glycogen.
- That better re-filling of liver glycogen improves the healthfulness of the diet.
How Do Sugars Perform at Refilling Glycogen?
Danny provides no citations for his claim that fructose “powerfully” refills glycogen. But Danny’s commenters help him out.
Daz, drawing upon a New York Times report, offers two studies [1] [2]. Cliff offers several more [3] [4]. Let’s see what these tell us.
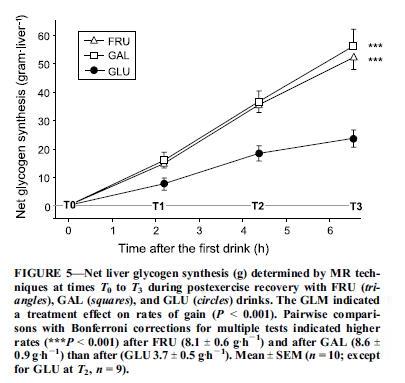
The first study, “Fructose and galactose enhance postexercise human liver glycogen synthesis” [1], looks at athletes depleted of liver glycogen by intense cycling, and assessed the effectiveness of three sugar drinks at replenishing liver glycogen. The three drinks were:
- 2/3 maltodextrin, 1/3 fructose;
- 2/3 maltodextrin, 1/3 glucose;
- 2/3 maltodextrin, 1/3 galactose.
Maltodextrin digests to glucose, so all three drinks are majority glucose. The athletes drink 275 calories of these drinks per hour for 6.5 hours after exercise. Galactose is non-toxic, but like fructose tends to be taken up by the liver.
Liver glycogen was measured every two hours with carbon-13 magnetic resonance imaging. Here were the results:
So 67% glucose / 33% galactose did the best, 67% glucose 33% fructose was close behind, and 100% glucose lagged.
Why does the 100% glucose drink underperform? One reason is that fructose and galactose, but not glucose, are preferentially targeted to the liver:
A factor of potentially larger magnitude in enhancing liver glycogen synthesis is the differential postabsorptive fates of fructose and glucose. Glucose is a relatively poor direct substrate for liver glycogen synthesis (24,27). Much of it is released from the liver into the systemic circulation to be stored as muscle glycogen (3,7). In contrast, fructose is primarily taken up by the liver … [1]
The second paper, “Superior endurance performance with ingestion of multiple transportable carbohydrates” [2], did not measure liver glycogen replenishment; instead, it gave its cyclists sugary drinks every 15 minutes throughout an intense 2-hr cycling test, and compared performance. Three different drinks were used: a 67% glucose 33% fructose drink, a 100% glucose drink, and a water-only control group. Performance was best with the 67% glucose 33% fructose drink, intermediate with the glucose drink, and worst with the water drink. The results suggest that the 67% glucose 33% fructose drink was better for liver glycogen replenishment, and that liver glycogen replenishment aided the cyclists’ performance.
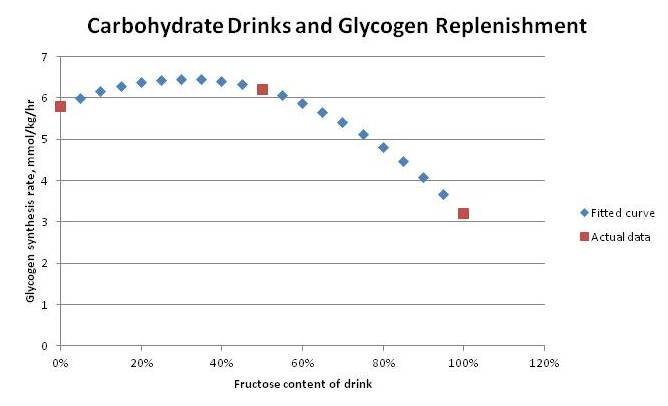
The third paper, “Effect of different post-exercise sugar diets on the rate of muscle glycogen synthesis” [3], isn’t available to me electronically, so we’ll have to work from the abstract. It looked at muscle glycogen, measured using biopsies (ouch!), rather than liver glycogen.
It first assessed the effect of different amounts of glucose. It found that 0.7 g/kg body weight of glucose given every 2 hours would maximize the rate of muscle glycogen synthesis. For an 80 kg man, that works out to 56 g or 224 calories of glucose per two-hour period, or 112 calories per hour. Above this amount, the rate of muscle glycogen synthesis is unchanged.
It then compared three formulations at this same 0.7 g/kg body weight dose: 100% glucose, 100% sucrose (50% glucose, 50% fructose), and 100% fructose. Muscle glycogen synthesis rates were:
- 5.8 mmol/kg/hr with 100% glucose
- 6.2 mmol/kg/hr with 50% glucose, 50% fructose
- 3.2 mmol/kg/hr with 100% fructose
If we fit a quadratic curve to these points, it predicts a peak rate of glycogen synthesis with 70% glucose, 30% fructose:
Athletes Agree: More Glucose Than Fructose
Of course, endurance athletes know that it’s beneficial to replenish glycogen during endurance events like marathons and triathlons.
Some authorities, including Tim Noakes, an exercise physiologist who has run over 70 marathons, believe that liver glycogen rather than muscle glycogen is the gating factor in marathon performance. From “The Science of Carbohydrate Loading” by David Peterson:
Remember also that muscle glycogen is committed to be used by muscle and cannot assist in maintaining blood sugar levels. Therefore should no additional carbohydrate be ingested during prolonged exercise, the task of maintaining blood glucose levels rests firmly on the liver’s glycogen stores and gluconeogenesis (the manufacturing of glucose from plasma amino acids). Oxidation of blood glucose at 70-80% VO2 max is about 1.0 g/min or about 60 g/hour. Therefore it can be predicted that even with full glycogen stores, a less conditioned athlete’s liver will be depleted of its carbohydrate within an hour and three quarters of continuous moderate intensity exercise. (Interestingly, the daily carbohydrate requirements of the brain and nervous system alone are enough to deplete the liver glycogen stores within 24 hours.) Once liver glycogen levels begin to drop and exercise continues the body becomes increasingly hypoglycemic (low blood sugar) mainly because blood glucose is depleted faster than it is replaced by gluconeogenesis. Professor Tim Noakes considers liver glycogen depletion and subsequent hypoglycemia to be the primary factors affecting fatigue and performance during extended duration races and especially in instances where muscle glycogen levels are low as well.
So marathoners and other endurance athletes will want to replenish liver glycogen as rapidly as possible during a race. What mix of sugars do they use?
The popular product is carbohydrate gels that can be swallowed at the same time water is taken. Here are the top carbohydrate gels sold on Amazon:
- Carb BOOM! Energy Gels – 24-Pack
consist of “a special blend of 22-25g of complex carbohydrates and just 2-4g of simple sugars to maximize energy delivery to working muscles.” The complex carbs are probably derived from starch and are 100% glucose, while the sugars probably contain some fructose. If the ratio is 2 g fructose in 25 g gel, then the composition will be 8% fructose 92% glucose.
- PowerBar Energy Gel, Double Caffeine, Tangerine, 1.44-Ounce Packets (Pack of 24)
consists of a 2:1 glucose to fructose blend, or 67% glucose 33% fructose.
- Clif Bar Shot gel, razz – 1.1oz (24/box)
list their primary ingredient as organic brown rice syrup, which is nearly 100% glucose.
So the sugar mix ranges from 67% glucose to 100% glucose. No product uses 50% fructose.
Presumably, athletes have done a great deal of personal experimentation and know that these ratios do, indeed, optimize the speed of glycogen replenishment.
When athletes have no need for speed, as when they are carb loading before a marathon, then they eat starches like pasta and bread, not sugar. So to maximize total glycogen status, regardless of speed of filling, a carb mix close to 100% glucose works just fine.
Glycemic Control
The fourth paper, “Acute fructose administration decreases the glycemic response to an oral glucose tolerance test in normal adults” [4], is about how a bit of fructose affects the glycemic response to an oral glucose tolerance test.
It showed that a 9% fructose 91% glucose test (7.5 g fructose, 75 g glucose) produced a lower glucose area under the curve and higher insulin response than a 100% glucose test. Here’s the glucose response:
In general higher insulin and lower glucose is healthier than the reverse, so this is considered an improvement.
Summary of the Data
What these papers show is:
- Glycogen replenishment proceeds the fastest with a mix of sugars consisting of about 70% glucose and 30% fructose or galactose.
- Although this wasn’t tested, we can guess that a mix of fructose and galactose would be more effective than fructose alone, since it seems that utilizing multiple carbohydrate pathways is what drives the speedier glycogen replenishment. So the fastest glycogen replenishment might occur with something like 70% glucose 15% fructose 15% galactose.
- Muscle glycogen replenishment is maximized with a carbohydrate intake of 100 calories per hour.
- Athletes agree with the research, using carb gel packs that contain typically 30-40 g carbs with a composition of 67% to 100% glucose, 0% to 33% fructose.
- Glycemic response to a large dose of carbohydrate may be improved by eating a 9% fructose 91% glucose mix.
From these data, I infer that for glycogen replenishment in liver or muscle, a PHD-style carb mix of 77% glucose, 15% fructose, 8% galactose is probably equal or superior to a Peat-style carb mix of 50% glucose, 50% fructose.
Conclusion
For athletes in the midst of a race, or in need of rapid recovery for a second race on the same day, speedy glycogen replenishment may be the endpoint to optimize. If so, they should eat a sugar drink composed of roughly 70% glucose and 30% fructose and galactose.
This is closer to PHD diet ratios than to Danny Roddy’s recommendation of orange juice or Travis Culp’s recommendation of soda!
But for others, speed of glycogen replenishment is hardly likely to be the parameter to optimize. There are unlikely to be significant benefits for non-athletes from replenishing glycogen 6.5% faster, as was found in the muscle glycogen study [3].
Speedier glycogen replenishment is almost the only known benefit to fructose consumption. It’s possible that low fructose doses, about 9% of carb calories (perhaps 2-3% of total calories), may improve glycemic control. This is a lower fructose fraction than is found in PHD, and far below the fructose fraction recommended by Danny and Travis.
Given the known risks of fructose consumption, especially with chronic intake at high doses or in conjunction with polyunsaturated fats, it seems prudent to err on the low side. It seems to me that the Peat-atarians have failed to provide any evidence at all in favor of a higher fructose intake than is provided by the fruits, berries, and sugary vegetables recommended by the Perfect Health Diet, save for athletes in the midst of a race or post-race recovery.
References
[1] Décombaz J et al. Fructose and galactose enhance postexercise human liver glycogen synthesis. Med Sci Sports Exerc. 2011 Oct;43(10):1964-71. http://pmid.us/21407126.
[2] Currell K, Jeukendrup AE. Superior endurance performance with ingestion of multiple transportable carbohydrates. Med Sci Sports Exerc. 2008 Feb;40(2):275-81. http://pmid.us/18202575.
[3] Blom PC et al. Effect of different post-exercise sugar diets on the rate of muscle glycogen synthesis. Med Sci Sports Exerc. 1987 Oct;19(5):491-6. http://pmid.us/3316904.
[4] Moore MC et al. Acute fructose administration decreases the glycemic response to an oral glucose tolerance test in normal adults. J Clin Endocrinol Metab. 2000 Dec;85(12):4515-9. http://pmid.us/11134101.













Recent Comments