I’d like to thank Patrick Timpone for a very enjoyable interview on The Morning Show at One Radio Network. Here is the MP3; I’m on for the second half of the show. You can find a zip file at the archive for October 13. Patrick’s producer Sharon tells me that she’s already benefited from our book:
I was following The Primal Diet and since I read the book, I’ve been allowing myself potatoes and rice and doing very very well on them among doing some other things you recommend.
Also, I’d like to thank Jimmy Moore once more for hosting his highly entertaining “safe starch” symposium (Jimmy’s original post; my response, here and at Jimmy’s). It was great to get the opportunity to explain ourselves to so many people in the low-carb and Paleo movements.
Jimmy is planning to try our diet for a week in November, which will be a good occasion for us to publish a 7-day meal plan. We’ll invite anyone who’s curious to try the diet along with Jimmy, and compare notes.
[1] Interesting posts this week:
Angelo Coppola on Latest in Paleo wonders if Denmark’s saturated fat tax will apply to mother’s milk. If so, it’s bad news for unemployed infants! (He also discusses the “safe starch” debate.)
I once knew a French astronomer who died from snorting cocaine while observing at 14,500 feet. Emily Deans makes me wonder: Did he have Crisco for dinner?
Stan the Heretic offers his mitochondrial dysfunction theory of diabetes. Peter Dobromylskyj and JS Stanton are also developing ideas along this line. Speaking of JS, his post this week has some great photos of Sierra wildflowers and reflections on the state of the Paleo community.
CarbSane partially confirms Dr. Ron Rosedale: eating carbs does raise leptin levels compared to eating fat, but it is a mild rise over an extended period of time, not a “spike.”
Beth Mazur explains why her bathroom door is always closed.
Chris Kresser discusses why chronic illness often generates a form of hypothyroidism, low T3 syndrome.
Joshua Newman knows how to flatter.
How solid is the case against Andrew Wakefield? Autism is certainly characterized by intestinal dysfunction, and Age of Autism notes that distinguished scientists are citing Wakefield’s work.
Richard Nikoley claims he doesn’t know the words to “Kumbayah.”
Seth Roberts points out that the Specific Carbohydrate Diet has been curing Crohn’s for 80 years, but still no clinical trial.
Jamie Scott, That Paleo Horse Doctor, asks: Why do horses get laminitis?
We’ve quoted vegetarian Dr. Michael Greger’s concerns about arsenic in eggs. I’m more concerned about soy protein in eggs.
Following Steve Jobs’s death, Tim asked for an opinion about the unconventional cancer therapies of Dr Mercola’s friend Nicholas Gonzalez. David Gorski, toward the end of a detailed examination of Jobs’s medical condition and treatment, links to his own claim that the Gonzalez protocol is “worse than useless.”
[2] Music to read by:
[3] Cute animal photo:
[4] Notable comments this week:
PeterC’s dad, who has diabetes, is doing well on our diet. Daniel’s stepdad had a similar experience.
Helen informs us that sweet potato intolerance may be due to raffinose.
Mario Iwakura gives us his infectious theory of diabetes. I think a lot of the cases of disrupted glucose regulation, where people get frequent hyperglycemic and hypoglycemic episodes, may be due to occult infections.
Dr Jacquie Kidd (who blogs at drjacs.com) has gotten some great advice from Jamie Scott.
Ellen tells us of cases of iodine supplementation controlling diabetes.
Ned is looking for grass-fed cowbells.
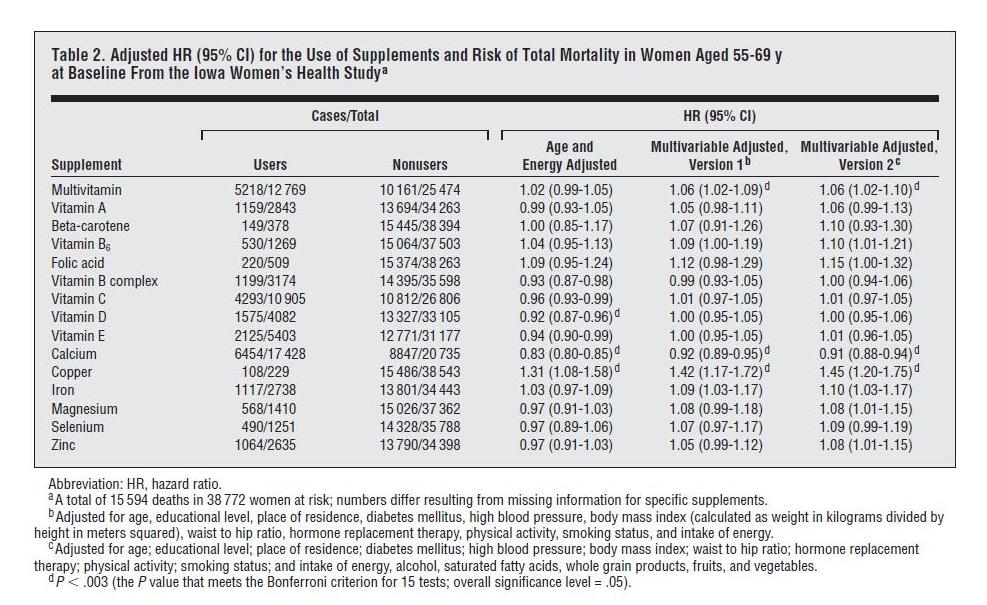
[5] Do Vitamins Kill?: An analysis of the Iowa Women’s Health Study came out this week, and it purported to show that nearly all supplements except calcium and vitamin D increased mortality, with iron being the worst. Oskar asked us to look into it, so we did.
The study followed a large number of women in Iowa, and queried them several times about supplement use. In 1986, the baseline, the women had an average age of 62 (range of 55 to 69) and 66% were taking supplements. By 2004, the surviving women had an average age of 82 and 85% were taking supplements.
Here is the data on overall mortality vs supplement use:
“Cases” are instances of someone dying. “HR” or hazard ratio is the likelihood of dying if you supplement divided by the likelihood of dying if you don’t. Note that all the hazard ratio estimates are “adjusted.”
Unadjusted Hazard Ratios
The left columns of the table give us death statistics and allow us to calculate raw hazard ratios, with no adjustment whatsoever. Seven of the supplements have unadjusted HRs below 1.00, eight have unadjusted HRs above 1.00. The 15 HRs average to 1.01. Without copper, which has an unadjusted HR of 1.17, they average to 0.998. In short, death rates among supplementers were almost identical to death rates among non-supplementers.
This is interesting because supplement usage rose rapidly with age. It was 66% at age 62 and 85% at age 82. Supplement users were, on average, older than non-supplement users. But mortality rises rapidly with age. So there should have been a lot more deaths among the supplement users, just because of their more advanced age.
The paper should have, but didn’t, report age-adjusted hazard ratios. Adjusting for age is very important, since mortality depends strongly on age, and so does supplement use. However, it’s obvious what the result of age-only adjustment would have been. Supplement usage would have shown a substantial reduction in the risk of dying.
Hazard Ratios Adjusted for Age and Energy Intake
The least-adjusted hazard ratios reported in the paper are adjusted for age and energy intake.
The energy intake adjustment is disappointing, because energy intake is affected by health: healthier people are more active and eat more, and obese people also eat more. Including indices of health as independent variables in a regression analysis will tend to mask the impact of the supplements on health, creating misleading results.
However, let’s go with what we have. Based on “Age and Energy Adjusted” hazard ratios, supplements generally decrease mortality. Nine of the fifteen supplements decreased mortality, five increased mortality. At the 95% confidence interval, five supplements decreased mortality, only one increased mortality.
Looking at the specific supplements, results are mostly consistent with our book analysis. Let’s start with the five that showed harm:
- Folic acid and iron – two nutrients we regard as dangerous and recommend not supplementing – both elevate mortality, as we would expect. Iron is particularly harmful, and should generally be avoided by women once they have stopped menstruating.
- Multivitamins slightly increase mortality, a result that has been found before and that we acknowledge in the book. This is probably due to (a) an excess of folic acid, (b) an excess of iron (if the women are taking iron-containing multis after menopause), (c) an excess of vitamin A (this is no longer the case – multi manufacturers have reduced the A content of vitamins in response to data – but in 1986-2004 most multis contained substantial amounts of A) which is harmful in women with vitamin D and/or K2 deficiencies (both extremely common, and D deficiency in this cohort is supported by the benefits of D and calcium in the study and the northerly latitude of Iowa) or (d) imbalances in other nutrients; for reasons of bulk multis tend to lack certain minerals, notably magnesium and calcium.
- Vitamin B6 is an anomaly, as we wouldn’t expect B6 to be harmful in moderation. I’m guessing B6 would have been taken to reduce high homocysteine and for this purpose would often have been taken along with folic acid, a harmful supplement. Also, B6 should be balanced by vitamin B12 and biotin, and may not have been.
Perhaps people with cancer were unaware that B6 promotes tumor growth;(UPDATE: See comments; I was misremembering studies, B12 and folic acid can promote tumor growth, but in other studies B6 looks protective against cancer) indeed, in the breakdown by cause of death in Table 3, B6 increases cancer mortality by 6%, but CVD mortality by only 1%. (Folic acid and vitamin A were other cancer-promoting supplements.) The harm from B6 was not statistically significant and I wouldn’t read much into it. - Copper is another anomalous result, but this was the least popular supplement, taken by only 229 women or 0.59%. Copper’s hazard ratios were dramatically affected by adjustment: in the raw data, mortality is only 17% higher among copper supplementers, but after age and energy adjustment it is 31% higher, and multivariable adjustment increases it substantially again. Clearly the effect of copper is highly sensitive to adjustment factors, indicating that copper was being taken by an unusual population. I think the hazard ratio for copper is impossible to interpret without knowing why these women were supplementing copper. If we knew their situation, there would probably be an appropriate adjustment that would make a huge difference in mortality. I would say the numbers are too small, the population too skewed, and the information too limited to draw any conclusion here.
Overall, I would interpret the nine that showed benefits as being highly supportive of micronutrient supplementation. The fact that vitamin A, vitamin B complex, vitamin C, vitamin D, vitamin E, calcium, magnesium, selenium, and zinc all reduced mortality suggests that a well-formulated multivitamin would likely have reduced mortality.
Hazard Ratios After Multivariable Adjustment
Now, what about the “Multivariable Adjusted” results, which were responsible for the headlines?
We have to keep in mind a famous aphorism from the mathematician John von Neumann:
With four parameters I can fit an elephant, and with five I can make him wiggle his trunk.
The multivariable adjustments use 11 parameters and 16 parameters respectively. Using so many parameters lets the investigators generate whatever results they want.
I don’t think it’s a coincidence that both multivariable adjustments substantially increased the hazard ratio of every single one of the 15 supplements. The 11-variable adjustment increased hazard ratios by an average of 7%, the 16-variable adjustment by an average of 8.2%.
Rest assured, it would have been easy enough to find multivariable adjustments that would have decreased hazard ratios for every single one of the 15 supplements.
I believe it verges on the unethical that the variables chosen include dangerous health conditions: diabetes, high blood pressure, and obesity. These three health conditions just happen to be conditions that are often improved by supplementation.
Anyone familiar with how regression analyses work will immediately recognize the problem. The adjustment variables serve as competing explanations for changes in mortality. If supplementation decreases diabetes, high blood pressure, and obesity, and through these changes decreases mortality, the supplements will not get credit for the mortality reduction; rather the decreased diabetes, blood pressure, and obesity will get the credit.
Imagine we had a magic pill that completely eliminated diabetes, obesity, and high blood pressure, and reduced mortality by 20%, with no negative health effects under any circumstances. But if regression analysis showed that non-diabetic, non-obese, and non-hypertensive people had 25% less mortality, then a multivariable adjusted analysis would show that the magic pill increased mortality. Why? Because the elimination of diabetes, obesity, and hypertension should have decreased mortality by 25% (the regression analysis predicts), but mortality was only decreased 20%, so adjusted for diabetes, obesity, and hypertension the magic pill must be credited with the additional 5% dead. The multivariable adjusted HR for the magic pill becomes 0.8/0.75 = 1.067.
Of course, what ordinary people want to know is: Will this magic pill improve my health? The answer to that would be yes.
What (too many) scientists want to know is: Which methodology for analyzing this magic pill data will get me grant money? That depends on whether the funding authorities are positively or negatively disposed toward the magic pill industry. Once you know that, you search for the 16-variable multivariable regression that generates the hazard ratios the authorities would like to see.
My take? Judging by the data in Table 2 plus corroborating evidence from clinical trials reviewed in our book, I would say that a well-formulated supplement program, begun at age 62, may increase the odds of survival to age 82 by something on the order of 5% to 10%. Perhaps not a magic pill; but worthwhile.
[6] Not the weekly video: An exceptional magic show:
[7] Shou-Ching’s Photo Art:
[8] Weekly video: A new tool for stroke recovery:














Recent Comments